ABSTRACT- The present study describes the hormonal regulation on morphogenesis in vitro in nodal segments of T. indica. The nodal explants, sterilized with 0.1% HgCl2, were cultured on Murashige and Skoog Medium enriched with various combinations and concentrations of plant hormones auxin and cytokinin to study the hormonal regulation on morphogenesis in vitro in T. indica. BAP at high concentration could not evoke any morphogenetic response in nodal explants. Calli formation at the basal part of nodal explants were noted on medium containing BAP (0.1 mg/L) and 2,4-D (5.0 mg/L). 0.1 mg/L BAP was found most effective in the shoot development of the T. indica. Rhizogenesis was observed on half-strength MS medium supplemented with 1.0 mg/l IAA and 0.1 mg/l NAA. The study may also be used mass-propagation and conservation of this medicinal plant species.
Key-words- Plant growth regulators, Morphogenesis in vitro, Tylophora indica, Rhizogenesis
INTRODUCTION-
T. indica (Burm. f.) Merrill, a member of Asclepiadaceae family, is an important medicinal plant species, which is used to cure various ailments such as bronchial asthma, bronchitis, rheumatism, inflammation, allergies and dermatitis [1-2]. The powdered leaves, stem and root contain several medicinally important alkaloids including tylophorine, tylophorinine [3]. Unfortunately, due to over exploitation for its bioactive constituents and lack of cultivation practices, the natural populations of this plant species has declined rapidly in last few decades. Recent developments in biotechnology provide new methods for conservation of threatened flora, using in vitro culture techniques. Through these techniques, mass-propagation of desired plant may be achieved by culturing explants on nutrient medium under in vitro conditions.
Plant hormones regulate many aspects of plant growth and development. Both auxin and cytokinin have been known for a long time to act either synergistically or antagonistically to control several significant developmental processes, such as the formation and maintenance of meristem. Therefore, in the present study, efforts were carried to screen the effects of various plant growth regulators on morphogenesis in vitro in T. indica. This type of study is highly beneficial to understand the role on various plant growth regulators on plant growth and development.
MATERIALS AND METHODS:
Plant material and surface sterilization: Nodal explants were collected during May 2011 to June 2011from healthy plants of T. indica growing in the plant nursery at the Jaipur National University, Jaipur (India). The excised nodal explants were washed thoroughly under running tap water to eliminate dust particles for 30 min and then sterilized in 0.1% HgCl2 solution for 4 - 6 min followed by thorough washing with sterile double distilled water.
Culture media and growth conditions: To study the hormonal regulation on morphogenesis in vitro, the sterilized nodal explants were cultured on Murashige and Skoog medium (MS media) [4] supplemented with various concentrations and combinations of auxin and cytokinin. The pH of the media was maintained 5.8 before autoclaving at 121 oC for 20 min. Cultured flasks inoculation were incubated at 25 ± 2 oC and 65 - 70% relative humidity with photoperiod of 16/8 h at 3000 lux intensity by florescent tubes.
RESULTS AND DISCUSSION
aspects of plant growth and development. The interaction between auxin and cytokinin is particularly important to control morphogenesis in plants. In the present study, nodal explants of T. indica did not show any morphogenetic response on media supplemented with various concentrations of Kinetin (Fig. 1 A). Shoot development was observed on low concentration of BAP (0.5 mg/L) (Fig. 1 B). Higher concentration of 6-Benzylaminopurine (BAP) showed inhibitory effect on shoot development. Similar, inhibitory effect of higher concentration of BAP on shoot development was observed in Gymemma sylvestre [5], Cunila galioides [6], Dictyospermum ovalifolium [7], and Codiaeum variegatum [8]. Auxins (2,4-D, IAA, IBA, NAA) alone, or in combination did not
trigger any response in nodal explants of T. indica. Development of compact calli was noted on medium supplemented with BAP (0.1 mg/L) and 2,4-D (5.0 mg/L) (Fig. 1 C). Low concentration of BAP (0.1 mg/L) was found most effective in shoot development of the T. indica (Fig. 1 D).
Rhizogenesis followed by callus formation was noted on medium fortified with 0.5 mg/L BAP and 5.0 mg/NAA (Fig. 1 E). Rhizogenesis was observed when nodal explants were cultured on ½ MS medium containing 1.0 mg/l IAA (Fig. 1 F). Frequency of rhizogenesis was enhanced when NAA was incorporated along with IAA (Fig. 1 G). ½ MS medium containing 1.0 mg/l IAA and 0.1 mg/l NAA produced high frequency root induction in nodal explants of T. indica.
<
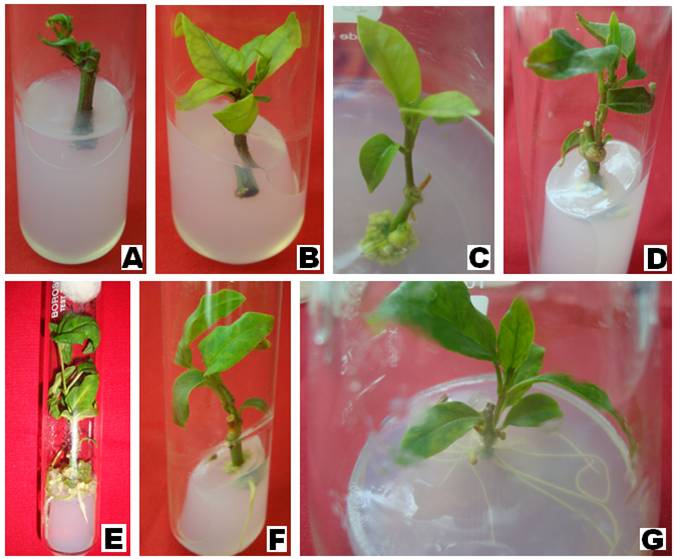
and 0.5 mg/L BAP (B); Rhizogenesis followed by shoot development on BAP (0.1 mg/L) and 2,4-D (5.0 mg/L) (C);
Elongated shoot on 0.1 mg/L, BAP (D) And rhizogenesis (E-F)t
CONCLUSION- The present study clearly demonstrates that BAP play an essential role in shoot development of the T. indica plant. Over-expression of BAP causes inhibitory effect on shoot formation. IAA and NAA at low concentrations act synergistically to promote rhizogenesis in vitro in the T. indica. The study will be useful to understand the hormonal basis of morphogenesis and plant development.
- REFERENCES
| International Journal of Life-Sciences Scientific Research (IJLSSR)
Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |
| How to cite this article: Soni V, Bhushan M: Hormonal Control of Morphogenesis in vitro in Nodal Segments of Tylophora indica. Int. J. Life. Sci. Scienti. Res, 2017; 3(4):1250-1252. DOI:10.21276/ijlssr.2017.3.4.25 Source of Financial Support:Nill Conflict of interest: Nil |