SSR Inst. Int. J. Life Sci., 8(2):
2990-2997,
March 2022
Cytoprotective
Effect of Dietary Squalene Supplementation on Experimentally Induced
Cardiomyopathy in Rats
Pallavi Srivastava1,2*, Agnivesh Gupta3
1Research and Development Cell, Bharathiar University,
Coimbatore-641046, Tamilnadu, India
2Department of Biochemistry, Sanskriti University, Chhata, Uttar
Pradesh-281401, India
3Department of Medical Laboratory
Technology, Sanskriti University, Chhata, Uttar Pradesh-281401, India
*Address for Correspondence: Dr. Pallavi
Srivastava, Associate Professor, Department of Biochemistry, Sanskriti University,
Chhata, Mathura-281401, Uttar Pradesh, India
E-mail: srivastavapallavi1914@gmail.com
ABSTRACT-
Background: Adriamycin is a
broadspectrum, potent, older chemotherapy drug and antineoplastic agent used in
the treatment of several cancers such as solid tumours, leukaemias, and
lymphomas, playing a major role in cancer chemotherapy. Long-term use of this
drug results in congestive heart failure and to overcome this effect dietary
squalene intake reduces the adverse effects of adriamycin-mediated
cardiotoxicity and cellular oxidative stress.
Methods:
The current study aims to investigate the cytoprotective effects of dietary
squalene supplementation on adriamycin-induced cardiomyopathy in rats in terms
of alterations in Troponin T, homocysteine, diagnostic marker enzymes, and
cardiac tissue histology.
Results:
The findings show that a 1.5 percent dose of dietary squalene supplementation
for 21 days reduced adriamycin-induced changes in homocysteine, troponin T,
diagnostic marker enzymes, and lesions in cardiac tissues.
Conclusion:
The outcomes of the study specified squalene's cytoprotective action which
stabilizes membranes against adriamycin-induced oxidative membrane degradation,
which is primarily responsible for heart cell irreversible necrosis.
Key Words:
Adriamycin, Cardiomyopathy, Diagnostic marker enzymes, Homocysteine,
Histopathology, Squalene, Troponin T
INTRODUCTION-
Adriamycin is a potent and broad-spectrum anticancer drug, used in numerous
cancer treatments such as solid tumours, leukaemias, and lymphomas. It plays
the foremost role in cancer chemotherapy and remains to be the first-line
antineoplastic drug. Adverse effects particularly dose-dependent
cardiomyopathies leading to potentially fatal congestive heart failure have led
to the limited clinical use of this drug [1].
After repetitive intake of Adriamycin
for several weeks or months, the chronic side effects develop which include
chronic cardiomyopathy cardiovascular dysfunctions, congestive heart failure
that are unchangeable, and also have a gloomy prognosis [2]. A delay
in the onset of adriamycin-induced cardiac dysfunction has been associated with
cardiomyopathy that becomes apparent 4-20 years later the completion of
chemotherapy in some patients [3].
The clinical features associated with chronic cardiomyopathy are a striking
decrease in blood pressure under 70/50 mmHg, tachycardia, dilatation of the
heart, and ventricular failure. Diagnostic enzyme markers like creatinine
phosphokinase, lactate dehydrogenase, and transaminases have also been stated
to increase markedly in these conditions. Distortion in cardiomyofibrils,
cytoplasmic vacuolization and increased number of lysosomes, and swelling of
mitochondria are some of the anomalies in ultrastructure connected with
adriamycin-induced cardiomyopathy [4,5].
Numerous metabolic and morphological
anomalies were observed within the cardiac tissue from laboratory animals after
the injection administration of Adriamycin, which is comparable to those
detected in human cardiomyopathy. The common symptoms presented include
impaired adrenergic stimulation, increased free radical formation, concealed
mitochondrial function, altered calcium homeostasis, infiltration of
inflammatory cells, and accumulation of fat [6-8]. Despite these opposing effects, Adriamycin is widely
used as an anticancer drug, and thus there will be higher chances of patients
getting cardiotoxic dosages of Adriamycin or even dying of this drug-related
toxic effect. Thus, there is a need to ascertain major risk factors, to
foretell those patients capable of bearing further doses of Adriamycin, and to
perhaps diminish cardiomyopathic aberrations becoming apparent.
In
modern medicines, many important drug discoveries have been started from the
bioactive substances available in nature. All over the world, this fact has
been channelled to biological screening programs for bioactive molecules which
led to research in this area. Traditional Indian medicine has many bioactive
molecules of interest and very little exploitation in this regard has happened.
To cure many cardiac disorders, the isoprenoid chemical, squalene, found in deep-sea shark liver
oil, was applied. Some amounts of squalene (0.1-0.7%)
are also available in the palm, olive, wheat-germ, and rice-bran oils. About 11% of the total surface fat
of human skin lipids is this hydrocarbon, which is also found in hair fat
dermoid cysts, cerumen, and sebum [9].
Squalene plays a foremost role in the upkeep of good health and holds antioxidant, antilipidemic, and membrane-stabilizing properties [10]. It partakes well in the synthesis of cholesterol, hormones, and vitamins and acts as a powerful endogenous antioxidant. To protect the skin from ultraviolet radiation, it is secreted in human sebum [11] and has been ascribed to possess cytoprotective [12] and anti-ageing properties [13]. Squalene has been found to inhibit the growth of cancer cells and neutralize carcinogens [14]. Squalene may be a good antidote, which can minimize the toxicity of unintentional drugs. Research revealed that squalene can act synergistically with marine polyunsaturated fatty acids to enhance myocardial dysfunction and a study has shown that the content of squalene, coenzyme Q10 (Co Q10), and vitamin E in skin surface lipids increases from childhood to adulthood and decreases again in old age, indicating that antioxidants can protect the body against exogenous oxidative damage and age-related diseases. The report shows that squalene is not toxic as a dietary supplement in food and capsules, and there is no problem after using squalene. In the current study, attempts have been made to examine the cytoprotective, antioxidant, hypolipidemic, and membrane stability properties of squalene in adriamycin-induced cardiomyopathy in rats.
MATERIALS AND METHODS
Place of Study- The study was conducted in July 2013, Department of Biochemistry, Research and Development, Bharathiar University, Coimbatore, Tamil Nadu, India.
Drugs and chemicals- Squalene (Refractive Index: 1.493; Specific Gravity:
0.853; Iodine Number: 344; Boiling Point: 240–245°C; Saponification Value: 30)
is a gift carefully prepared by Dr. T.K. Thankkappan, Chief Scientist, ICAR-
Central Institute of Fisheries Technology, Cochin 682029, India. Get
Adriamycin, lactate, and aspartate from Sigma Chemical Company, St. Louis,
Missouri, United States. Other chemicals purchased are of analytical quality.
Animals- Male Wistar rats weighing 120-150 g are kept under standard
environmental conditions. The animals receive standard pellet feed and free
drinking water from M/s Sai Foods in Bangalore, India.
Experimental protocol- Four groups of six rats in each group were used for
the study, and experiments were conducted by the rules of the Committee for
Control and Supervision of Animal Experiments in New Delhi, India (CPCSEA).
Group I and Group III animals were fed commercial feed supplemented with 1.5%
coconut oil for 21 days. For Group II and Group IV, animals were fed commercial
feed supplemented with a 1.5% level of squalene for 21 days. Intraperitoneal
(IP) injection of Adriamycin [15 mg/kg (IP) in 6 equal injections within 2
weeks] was injected into group III and group IV animals to induce
cardiomyopathy. Physiological saline was i.p. injected in control animals
(Group I and Group II).
After the completion of the experiment, the experimental rats were killed
and for collecting the blood and the separation of plasma, an anticoagulant was
added to the blood. Heart tissue was removed and immediately washed with frozen
isotonic saline. A part of the tissue was fixed in 10% buffered formalin for
histopathological interpretation. Troponin T is measured using an
electro-chemiluminescence immunoassay. A microtiter plate assay kit (Diazyme
Laboratories) was used to determine the plasma homocysteine concentration
(tHcy). Aspartate aminotransferase (ALT), alanine aminotransferase (AST),
creatine phosphokinase (CPK), and lactate dehydrogenase (LDH) it is measured in plasma.
Statistical Analysis- Results
are expressed as mean±SD. Significant ANOVA was used for multiple comparisons
using Duncan's multiple comparison tests. A value of p<0.05 is considered statistically
significant. All data is analyzed with the help of the statistical software
package SPSS 10.0 for Windows.
RESULTS
Cytoprotective effect of
squalene against adriamycin-induced cardiomyopathy in rats- The animals of groups I and III were fed with
commercial feed supplemented with 1.5% coconut oil for 21 days, and the animals
of groups II and IV were fed with commercial feed supplemented with 1.5%
squalene for 21 days. Group III and group IV animals were injected
intraperitoneally (i.p.) with Adriamycin [15 mg/kg (i.p.) In 6 equal injections
over 2 weeks] to induce cardiomyopathy. Value expressed: troponin T-ng/ml;
homocysteine μmol/l. The results are the Mean±SD of 6 animals; one-way
analysis of variance; Duncan's multiple comparison test. Values with different
letters (a, b, c) are significantly different from each other (p<0.05)
(Table 1).
Table
1: The
level of troponin T and homocysteine in the plasma of the rat
aberration control group and experimental group.
|
Parameters |
Group
I |
Group
II |
Group
III |
Group
IV |
|
Troponin
T |
0.05±0.01a |
0.05±0.01a |
1.85±0.09b |
0.11±0.01c |
|
Homocysteine |
4.82±0.28a |
4.96±0.33a |
14.32±1.27b |
5.48±0.46a |
Diagnostic marker enzymes- Animals in groups I and III were fed commercial feed
supplemented with 1.5% coconut oil for 21 days, and animals in groups II and IV
were fed commercial feed supplemented with 1.5% squalene for 21 days. Group III
and Group IV animals were injected intraperitoneally (i.p.) with doxorubicin
[15 mg/kg (i.p.) in 6 equal injections over 2 weeks] to induce cardiomyopathy.
The numerical value indicates the number of micromoles of ALT, AST, and LDH of pyruvate
released/h/l; the creatine formed by CPK μmol/h/l. The results are the
mean±SD of 6 animals; one-way analysis of variance; Duncan's multiple
comparison test. Values with different letters (a, b, c) are
significantly different from each other (p<0.05) (Table
2).
Table 2: Plasma levels of alanine
aminotransferase (ALT), aspartate aminotransferase (AST), lactate dehydrogenase
(LDH), creatine phosphokinase (CPK), and AST / ALT ratio in the
groups of normal and experimental rats
|
Parameters |
Group I |
Group II |
Group III |
Group IV |
|
ALT |
115.3±8.24a |
109.8±7.45a |
256.3±16.8b |
134.1±11.2c |
|
AST |
132.2±9.12a |
126.2±8.93a |
294.6±18.5b |
161.7±12.4c |
|
LDH |
155.6±11.4a |
143.1±10.2a |
318.7±21.2b |
179.2±15.6c |
|
CPK |
137.3±10.5a |
127.9±9.74a |
278.3±17.6b |
164.2±10.9c |
|
AST/ALT
Ratio |
1.18±0.02
a |
1.16±0.02a |
1.12±0.01b |
1.20±0.02c |
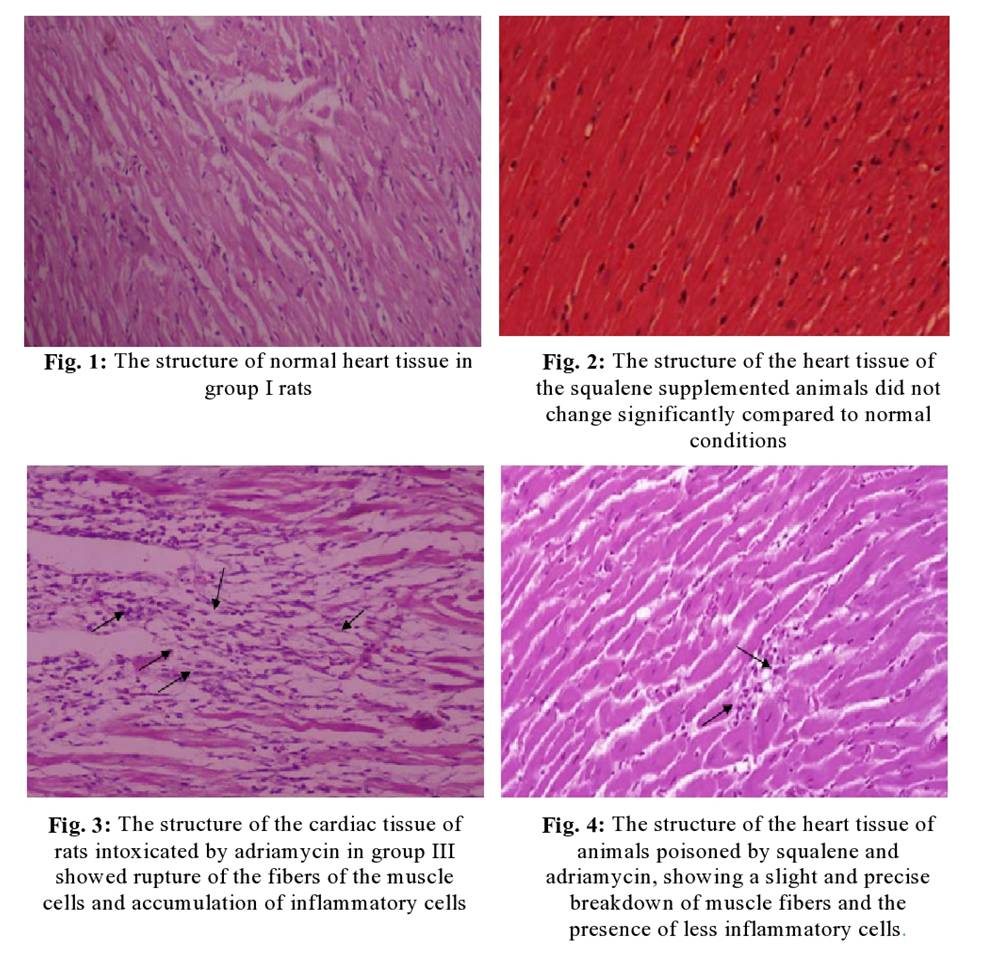
Histopathological
Studies- Histological annotations were made to the myocardial
tissues of normal and experimental groups of animals to confirm the cytoprotective
activity of squalene against adriamycin-induced cardiomyopathy. Fig. 1 shows
disclosed regular myofibrillar architecture with striations, bifurcated
appearance, and permanency with contiguous myofibrils by microscopic
examination of heart tissue slices of Group I normal rats. Squalene received
histological inspections on the heart tissue of normal rats of Group II in Fig.
2 did not display any significant variations when compared with control rats,
showing that it does not per se have any adversative effects. Fig. 3 showed
architectural abnormalities in cardiac tissue sections of Group III rats after
administration of Adriamycin as compared to the normal Group I control rats
such as mild to diffused cloudy swelling, focal vacuolar disintegration, and
occasional pericentral infiltration of round cells. The light microscopical
analysis of Group IV squalene supplemented rats on the heart tissue sections
was shown in Fig. 4, which exhibited regular architecture of myofibrillar
striations, branched appearance, and continuity with adjacent myofibrils as
compared to the normal Group I control rats.
DISCUSSION- In the myocardium, troponin T is a key regulator of the
contraction and relaxation process observed with actin filaments. One of the
main indicators of myocardial dysfunction in the systemic circulation is
elevated troponin T levels. A study showed that troponin T is an effective
biomarker for sensitive detection in experimental animals, and it has specific
damage to the heart [15].
In
this study, compared with the control animals in group I, the level of troponin
T in the plasma of rats in group III given adriamycin was significantly
increased (p<0.05). This is consistent with previously
established studies, which showed that the detection of troponin in the
systemic circulation is more sensitive and specific for assessing the severity
of cardiomyopathy-induced adriamycin Markers [16]. Dietary supplementation
of squalene significantly reduced (p<0.05) the release of adriamycin-induced
troponin T from the heart tissue into the systemic circulation, indicating its
protective effect on the myocardial membrane system. This may be done by
maintaining a delicate balance of cardiomyocyte tension.
Squalene present in the
cell and subcellular membranes has the function of regulating cell volume and
regulating the elasticity of the plasma membrane. Since regulation of cell volume
affects cell function, the presence of squalene in the membrane plays an
important role in protecting the myocardium from necrotizing lesions. As in
previous studies, isoprenoid squalene molecules can avoid the severe osmotic
pressure changes associated with apoptosis. In the process of methionine
metabolism, thiols and homocysteine containing cytotoxic 4-carbon
alpha-amino acids are produced, which can impair the function of coronary
microvascular dilators or promote smooth muscle proliferation [17], thrombosis, platelet
activation [18] and
endothelial abnormalities [19].
Homocysteine is a gene in endothelial cells [20]. A powerful mediator of inflammation progression and elevated
homocysteine levels are associated with interleukins in monocytes
[21]. The increase in
production is related to the positive regulation of vascular cell adhesion
molecules [22].
The increased risk of cardiovascular disease independent of classic
risk factors is even associated with mild hyperhomocysteinemia [23]. In the current study, compared with group
I control rats, the level of homocysteine in the plasma of group
III animals taking adriamycin significantly increased, which is different from
the previous A published study is consistent [24]. The exact pathophysiological role of
homocysteine-induced cardiomyopathy is unclear, and there is a large amount of
research evidence supporting the role of homocysteine in the
development of myopathy aberrations. Compared
with group III cardiomyopathy-induced animals, dietary squalene supplementation
greatly reduced the plasma homocysteine content of group IV rats. This may be
due to the inhibition of monocyte/macrophage-derived interleukin production,
which triggers the firm adhesion of rolling monocytes to the vascular endothelium,
which is the cause of atherosclerosis [25]. A study conducted in
2002 showed that the lipophilic inhibitors cerivastatin, fluvastatin, and
HMGCoA reductase reduce cardiovascular risk and atherosclerosis through
non-lipid mechanisms (such as inhibition of interleukin expression) plaque
fragility [26]. The lipophilic nature of squalene is more than
statins thereby increasing their permeability to vascular smooth muscle cells
and thus inhibiting the production of both interleukin and homocysteine like
HMG-CoA reductase inhibitor.
The damaged
myocardium released diagnostic marker enzymes (CPK, CPK-MB, LDH, AST, ALT, and alkaline phosphatase) into the systemic circulation
after adriamycin-induced myocardial injury. The number of damaged
myocytes present in
the myocardium is directly related to circulatory levels of marker enzymes in plasma. These
enzymes are considered to be the best markers of myocardial damage because they
have specific and catalytic effects on all other biomolecules leaking from
damaged heart tissue. The levels
of diagnostic marker enzymes concentration in plasma showed a significant (p<0.05) rise in
Group III adriamycin-administered animals as compared to Group I control rats.
This is consistent with the results of a previous study [27], which clarified the intensity of necrotic
damage to the myocardial cell membrane caused by adriamycin. Release of the
labelled enzyme reveals nonspecific abnormalities in plasma membrane integrity
and permeability in response to adrenergic stimulation. The cytoprotective activity of squalene was
confirmed, because compared with group III, consumption of squalene by oral
route significantly offset (p<0.05) the increase in the intensity of
diagnostic marker enzymes in the plasma of group IV animals caused by
adriamycin mouse. Squalene is lipophilic and can be combined with any other
lipophilic drugs, such as antipyrine, vitamin E, and nifedipine [28].
Regarding the degree of lipophilicity, lipophilic
β-blocking molecules insert into the lipid bilayer and stabilize the
muscle cell membrane. Since then, it
is conceivable that squalene may also extend the sustainability of myocardial
cell membrane necrosis damage through membrane stabilization. In the myocardial
tissues of normal and experimental animals, histological annotations were made
to verify the cytoprotective activity of squalene against adriamycin-induced
cardiomyopathy.
Microscopic study of cardiac tissue sections from normal Group I rats revealed the structure of regular myofibrils with stripes, the appearance of bifurcations, and the permanence of continuous myofibrils. However, compared to normal control group I rats, adriamycin administration can cause structural abnormalities, such as mild to diffuse cloudy swelling, focal vacuolization, and group III rats heart tissue sections Occasionally infiltrates around the centre of round cells. Congestion, dilation of the hepatic sinusoids, occasional cell proliferation, central necrosis, and fibrous hyperplasia in the portal area were also observed. These structural irregularities may be due to the drop in oxygen supply and increased parallel wall stress. Current observations corroborate previously reported research [29,30] indicating that adriamycin-induced histological changes are noted in the left ventricular subendocardium.
Therefore, in the current study, light microscope analysis of the fourth group of rat heart tissue sections supplemented with squalene showed the regular structure of the myofibril stripes, the appearance of branches, and the continuity with the adjacent myofibrils. The myocardial fibers are well protected and are similar to the myocardial fibers of control rats, representing the cytoprotective effect of squalene. Previous research is pointed out that oral squalene improves the morphological changes of the heart caused by isoproterenol through its membrane-stabilizing properties [31]. A histopathological study demonstrated that squalene intake can protect experimental animals from cyclophosphamide-induced tissue damage [32]. Compared to control rats, histological examination of heart tissue from normal rats that received squalene alone (group II) did not show any significant change, indicating that it did not have any adverse effects on its own.
Squalene
dietary supplementation has a protective effect on adriamycin-induced
cardiomyopathy in rats. The overall cardioprotective effect of squalene may be
related to its ability to stabilize the membrane against the deterioration of
the oxide film induced by Adriamycin, which is the main cause of irreversible
necrosis of cardiomyocytes.
CONCLUSIONS-
Adriamycin is a powerful extensively used anticancer drug that plays an
important role in cancer chemotherapy. The clinical efficacy of this drug is
very limited as it causes continuous cardiomyopathy or congestive heart failure
in cancer patients. The heart tissue has particularly become toxic which is a
major cause of morbidity and mortality due to its complex etiology. Despite its
side effects, Adriamycin is widely used and Adriamycin is the best-selling
anticancer drug in the world. A better understanding of the underlying
mechanisms of adriamycin-induced cardiomyopathy has led to the development of
new cardioprotective therapies. Correcting possible myocardial dysfunction can
be a useful and practical principle in the treatment of patients with
cardiomyopathy. Squalene is an isoprenoid molecule used in Indian folk
remedies, present in large quantities in cod liver oil and extracted from
deep-sea sharks to treat cardiovascular disease. It has been reported to have
important membrane stabilizing properties.
The
target of the present study is to examine the cytoprotective effects of dietary
supplementation of squalene against adriamycin-induced cardiomyopathy in rats,
an animal model for cardiomyopathy of human beings, and the results correlate
the statement by maintaining the levels of diagnostic markers at near normalcy
and through inhibiting the formation of lesions in the cardiac tissue in the
experimental rats. This research study relates to the ability of a natural
bioactive substance i.e., dietary squalene intake in reducing the adverse
effects of adriamycin-mediated cardiotoxicity and cellular oxidative stress.
CONTRIBUTION OF AUTHORS
Research concept- Dr. Pallavi Srivastava
Research design- Dr.
Pallavi Srivastava and Mr. Agnivesh
Supervision- Dr.
Pallavi Srivastava
Materials- Dr.
Pallavi Srivastava and Mr. Agnivesh
Data collection- Dr.
Pallavi Srivastava and Mr. Agnivesh
Data analysis and Interpretation- Dr.
Pallavi Srivastava
Literature search- Dr. Pallavi Srivastava
Writing article- Dr.
Pallavi Srivastava and Mr. Agnivesh
Critical review- Dr.
Pallavi Srivastava
Article editing- Dr.
Pallavi Srivastava and Mr. Agnivesh
Final
approval- Dr. Pallavi Srivastava and
Mr. Agnivesh
REFERENCES
1. Mingyan
E, Hongli L, Shufeng L, Bo Y. Effects of pyrrolidine dithiocarbamate on
antioxidant enzymes in cardiomyopathy induced by adriamycin in rats. Cardiol.,
2008; 111(2): 119-25.
2. Quiles
JL, Huertas JR, Battino M, Mataix J, Ramirez-Tortosa MC. Antioxidant nutrients
and adriamycin toxicity. Toxicol., 2002; 180: 79–95.
3. Simsir
SA, Lin SS, Blue LJ, Gockerman JP, Russell SD, et al. Left ventricular assist device as destination
therapy in doxorubicin-induced cardiomyopathy. Ann Thorac Surg., 2005; 80:
717–19.
4. Zeidán
Q, Strauss M, Porras N, Anselmi G. Differential long-term subcellular responses
in heart and liver to adriamycin stress. Exogenous L-carnitine cardiac and
hepatic protection. J Submicrosc Cytol Pathol., 2002; 34(3): 315-21.
5. Sardão
VA, Oliveira PJ, Holy J, Oliveira CR, Wallace KB. Morphological alterations
induced by doxorubicin on H9c2 myoblasts: nuclear, mitochondrial, and
cytoskeletal targets. Cell Biol Toxicol., 2009; 25(3): 227-43.
6. Subashini
R, Ragavendran B, Gnanapragasam A, Yogeeta SK, Devaki T. Biochemical study on
the protective potential of Nardostachys jatamansi extract on lipid profile and
lipid metabolizing enzymes in doxorubicin intoxicated rats. Pharmazie., 2007;
62: 382-87.
7. Catalá
A, Zvara A, Puskás LG, Kitajka K. Melatonin-induced gene expression changes and
its preventive effects on adriamycin-induced lipid peroxidation in rat liver. J
Pineal Res., 2007; 42(1): 43-49.
8. Gnanapragasam
A, Yogeeta S, Subhashini R, Ebenezar KK, Sathish V, et al. Adriamycin induced
myocardial failure in rats: protective role of Centella Asiatica. Mol Cell
Biochem., 2007; 294(1-2): 55-63.
9. Passi
S, Pita OD, Puddu P, Littarru GP. Lipophilic antioxidants in human sebum and aging.
Free Radic Res., 2002; 36: 471-477.
10. Farvin
KHS, Anandan R, Kumar SHS, Shiny KS, Sankar TV, et al. Effect of squalene on
tissue defense system in isoproterenol-induced myocardial infarction in rats.
Pharmacol Res., 2004; 50: 231-36.
11. Kohno
Y, Egawa Y, Itoh S, Nagaoka S, Takahashi M, et al. Kinetic study of quenching
reaction of singlet oxygen and scavenging reaction of free radical by squalene
in n-butanol. Biochim Biophys Acta., 1995; 1256: 52-56.
12. Dhandapani
N, Ganesan B, Anandan R, Jeyakumar R, Rajaprabhu R, et al. Synergistic effects
of squalene and polyunsaturated fatty acid concentrate on lipid peroxidation
and antioxidant status in isoprenaline-induced myocardial infarction in rats.
Afr J Biotechnol., 2007; 6: 1021-27.
13. Buddhan
S, Sivakumar R, Dhandapani N, Ganesan B, Anandan R. Protective effect of
dietary squalene supplementation on mitochondrial function in liver of aged
rats. Prostaglandins Leukot Essent Fatty Acids, 2007; 76: 349-55.
14. Farvin
KHS, Rajesh R, Surendra Raj A, Anandan R, Sankar TV. Squalene- a gift from the
sea. Science India, 2009; 12: 20-24.
15. O'Brien
PJ, Landt Y, Ladenson JH. Differential reactivity of cardiac and skeletal
muscle from various species in a cardiac troponin I immunoassay. Clin Chem.,
1997; 43: 2333-38.
16. Kismet
E, Varan A, Ayabakan C, Alehan D, Portakal O, et al. Serum troponin T levels
and echocardiographic evaluation in children treated with doxorubicin. Pediatr
Blood Cancer, 2004; 42(3): 220-24.
17. Liu
X, Shen J, Zhan R, Wang X, Wang X, et al. Proteomic analysis of homocysteine
induced proliferation of cultured neonatal rat vascular smooth muscle
cells.Biochim Biophys Acta., 2009; 1794(2): 177-84.
18. Dionisio
N, Jardín I, Salido GM, Rosado JA. Homocysteine, intracellular signaling, and
thrombotic disorders. Curr Med Chem., 2010; 17(27): 3109-19.
19. Noll
C, Lameth J, Paul JL, Janel N. Effect of catechin/epicatechin dietary intake on
endothelial dysfunction biomarkers and proinflammatory cytokines in the aorta
of hyperhomocysteinemic mice. Eur J Nutr., 2013; 52(3): 1243-50.
20. Zhang
D, Chen Y, Xie X, Liu J, Wang Q, et al. Homocysteine activates vascular smooth
muscle cells by DNA demethylation of platelet-derived growth factor in
endothelial cells. J Mol Cell Cardiol., 2012; 53(4): 487-96.
21. Korokin
MV, Pokrovskiy MV, Novikov OO, Gudyrev OS, Gureev VV, et al. A model of
hyperhomocysteine-induced endothelial dysfunction in rats. Bull Exp Biol Med.,
2011; 152(2): 213-15.
22. Van
den Kommer TN, Dik MG, Comijs HC, Jonker C, et al. Homocysteine and
inflammation: predictors of cognitive decline in older persons? Neurobiol
Aging, 2010; 31: 1700-09.
23. Sternberg
Z, Leung C, Sternberg D, Li F, Karmon Y, et al. The prevalence of the classical
and non-classical cardiovascular risk factors in multiple sclerosis
patients.CNS Neurol Disord Drug Targets, 2013; 12(1): 104-11.
24. Mohamed
HE, El-Sw Estevez efy SE, Hagar HH. The protective effect of glutathione
administration on adriamycin-induced acute cardiac toxicity in rats. Pharmacol
Res., 2000; 42: 115–21.
25. Gerszten
RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, et al. MCP-1 and IL-8 trigger
firm adhesion of monocytes to vascular endothelium under flow conditions.
Nature, 1999; 398: 718-23.
26. Ito
T, Ikeda U, Yamamoto K, Shimada K. Regulation of interleukin-8 expression by
HMG-CoA reductase inhibitors in human vascular smooth muscle cells.
Atherosclerosis, 2002; 165, 51-55.
27. Gnanapragasam
A, Ebenezar KK, Sathish V, Govindaraju P, Devaki T. Protective effect of Centella Asiatica on
antioxidant tissue defense system against Adriamycin-induced cardiomyopathy in
rats. Life Sci., 2004; 76: 585–97.
28. Rajesh
R, Lakshmanan PT. Antioxidant defense of dietary squalene supplementation on
sodium arsenite-induced oxidative stress in rats. Int J Biomed Pharm Sci.,
2008; 2: 98-102.
29. Karagoz
B, Suleymanoglu S, Uzun G, Bilgi O, Aydinoz S, et al. Hyperbaric oxygen therapy
does not potentiate doxorubicin-induced cardiotoxicity in rats. Basic Clin
Pharmacol Toxicol., 2008; 102(3): 287-92.
30. Christiansen S, Perez-Bouza A, Schälte G,
Hilgers RD, Autschbach R. Selective left ventricular adriamycin-induced
cardiomyopathy in the pig. J Heart Lung Transplant, 2008; 27(1): 86-92.
31. Farvin
KHS, Kumar SHS, Anandan R, Mathew S, Sankar TV, et al. Supplementation of
squalene attenuates experimentally induced myocardial infarction in rats. Food
chem., 2007; 105: 1390-95.
32. Senthilkumar
S, Devaki T, Manohar BM, Babu MS. Effect of squalene on
cyclophosphamide-induced toxicity. Clin Chim Acta., 2006; 364(1-2): 335-42.