1Plant Bioenergetics and Biotechnology Laboratory, Department of Botany, Mohanlal Sukhadia University, Udaipur, India,
2Department of Botany, University of Rajasthan, Jaipur, India
*Address for Correspondence: Dr. Vineet Soni, Assistant Professor, Department of Botany, Mohanlal Sukhadia
University, Udaipur, India
Received: 18 March 2017/Revised: 28 May 2017/Accepted: 23 June 2017
ABSTRACT-The effects of water deficit induced by withholding water in soil pots were examined on the activities of various key enzymes i.e. nitrate reductase, peroxidase, acid phosphatase, a-amylase and invertase in Commiphora wightii. Drought stress induced decrease in the activities of nitrate reductase, peroxidase, a-amylase and invertase was observed in leaves of C. wightii. The decreased activity of peroxidase enzyme in C. wightii plants under water stress condition indicates that the plant is capable of maintaining growth vigour despite adverse conditions. On the other hand, acid phosphatase activity increased continuously in the leaves of C. wightii plants subjected to water stress. The results clearly indicate that regulation of enzymatic activity under drought is an essential biochemical process, which prevents the plants from drought-induced damage.
Key-words- Drought, Commiphora wightii, Nitrate reductase, Peroxidase, Acid phosphatase, a-amylase, Invertase
Drought is one of the important biomass-limiting stress factors which affect practically every aspect of plant growth and metabolism [1]. Plant’s responses to water deficit condition depend upon various factors such as duration and degree of stress, growth stage and time of stress exposure. During drought the water potential (?), relative water content (RWC) and net photosynthetic CO2 fixation (A) significantly decrease [2]. The stomatal closure is among the first responses to the water stress, and is assumed to be the main cause of impaired photosynthesis, since the stomatal closure limits CO2 availability to the mesophyll. On the other hand, the limitation of CO2 fixation during drought is also influenced by the diffusion of CO2 from the intercellular spaces to chloroplasts [3], and by other metabolic factors such as changes in the activity of ribulose-1,5-bisphosphate-carbosilase and perturbed re-generation of ribulose-1,5-bisphosphate etc [3].
Commiphora wightii (Arnott) Bhandari (Burseraceae) is a slow growing, highly branched, and critical en-dangered plant that grows wild in the arid and semi-arid regions of India, Pakistan and tropical regions of Africa.
In India, the plant is wide spread in dry and arid region of Rajasthan and Gujarat states. Plants grown in such arid and semi-arid regions often encounter various stress conditions such as high temperature, high wind regime, high light intensity, drought etc. Against drought, plants adapt themselves by different mechanisms including change in morphological and developmental pattern as well as physiological and biochemical responses [4]. Adaptation to drought is associated with metabolic adjustments that lead to the modulation of different enzymes. It has been hypothesized that these particular changes induced under water deficit conditions enables the plant to endure drought stress. Therefore, in the present instigations, efforts were carried out to understand the biochemical basis of drought tolerance in C. wightii plants.
MATERIALS AND METHODS
Plant materials and Drought treatment:- Two year old vegetatively propagated plants of C. wightii (Fig. 1 A) growing in pots at research nursery, Department of Botany, University of Rajasthan, Jaipur, India during March 2005 were used for the biochemical studies. The plants were divided into two sets (each of four plants), out of which one set was subjected to water stress by withholding of water supply till wilting symptoms appeared, while the second set was watered regularly and served as a control.
Sample preparation- Biochemical analyses of enzymes were carried out in the leaves as the leaves were devoid of resinous compounds, which interfere with biochemical estimations. The samples were collected regularly at an interval of three days till day 15. The leaves were homogenized in pre-chilled mortar and pestle using appropriate buffers. The homogenate was centrifuged in high-speed centrifuge (KR 20000 T, KUBUTA, Japan) at 10,000 rpm for 20 minutes. The supernatant thus collected was used for enzyme assay and protein estimation. Results were averages of two replicates.
Enzyme assay- A method given by Jaworski [5] was used for assaying nitrate reductase activity. Guaiacol H2O2 method was used for assaying the activity of peroxidase [6]. The activity of acid phosphatase was assayed by using p-nitrophenyl phosphate as a substrate [7] and Bernfeld's method [8] was used for assaying the activity of a- amylase. A modified method of Harris and Jeffcoat [9] was used to determine the activity of acid invertase.
RESULTS AND DISCUSSION
Metabolic changes in response to water stress also include reduction in photosynthetic activity and accumulation of organic acids, such as malate, citrate and lactate accompanied by accumulation of proline, sugars and betaine [10-11]. Exposure of plants to low water potential often leads to loss of cell turgor and then plants undergo osmotic adjustments by the rapid accumulation of abscisic acid (ABA) and osmoprotectants [12]. In the present investigation, the activities of enzymes (nitrate reductase, peroxidase, acid phosphatase, a-amylase and invertase) and level of cellular metabolites (proteins and proline) were ana-lyzed in C. wightii plants subjected to water deficit condition.
Nitrate reductase, which is involved in reduction of NO-3 to NO-2, is known to be highly sensitive to all types of stresses, particularly salt and drought stresses. In present study, a remarkable decline in the nitrate reductase activity was observed after 3rd day of water deprivation (Fig. 1 B). On day 15 of water deprivation, the activity of nitrate reductase enzyme decreased to 15.6 % (± 2.02) of initial level. Similarly Inhibition of nitrate reductase under moisture stress has been reported by several workers in clusterbean [13], rice [14], corn [15], wheat [16], and Sorghum [17].
Peroxidase activity decreased continuously in the leaves of C. wightii plants subjected to water deficit conditions (Fig. 1 C). On day 15 the activity of this enzyme decreased to 30.7 % (± 1.421) of initial level. The variation in peroxidase activity in the plants growing under normal irrigated condition was insignificant. The decrease in activity is ascribed to as a change in the structural proteins [18]. In general, the peroxidase activity is inversely correlated with the plants growth rates. The decreased activity of peroxidase enzyme in plants undergo water stress condition indicates that the plant is capable of maintaining growth vigour despite adverse conditions.
Acid phosphatase is known to maintain a certain level of inorganic phosphate in plant cells under stress [19]. In present study, acid phosphatase activity increased continuously in the leaves of C. wightii plants subjected to water stress (Fig. 1 D). Induction of acid phosphatase activity may be due to fact that under conditions of water stress, growth is restricted and delivery of phosphate is impaired, resulting in the activation of the cellular phosphatases that release soluble phosphate from its insoluble compounds inside or outside the cells [20]. Olmos and Hellin [21] observed that acid phosphatase under salt and water stress maintained a certain level of inorganic phosphate which could be co-transported with H+ along a gradient of proton motive force.
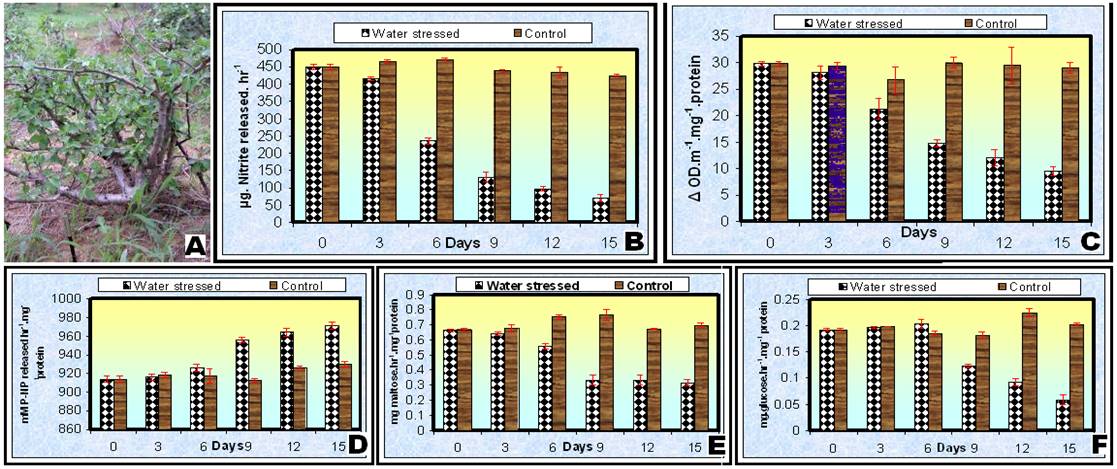
(C) Acid phosphatase, (D) a-amylase, (E) Invertase, (F) C. wightii under drought stress
Activity of a-amylase declined marginally till day 6 and declined sharply thereafter in the guggul plants subjected to water stress. The a-amylase activity in non-stressed plants increased marginally during first nine days (Fig. 1 E). Similarly results were also observed in pearl millet [22]. Invertase activity initially increased till day 6 and then declined sharply with an increasing water starvation (Fig. 1 F). The initial increase in invertase activity in plants indicates its involvement in water stress tolerance mechanism. Thind and Malik [23] also observed an increase in acid invertase activity in wheat at low stress levels.
CONCLUSIONS
In the present investigations, biochemical basis of drought tolerance in the succulent plant C. wightii was studied. Biochemical analysis indicates that plants are quite resistant and well developed to endure drought stress by modulating the key enzymes such as enzymes nitrate reductase, peroxidase, acid phosphatase, a-amylase and invertase. The study will be highly beneficial in development of transgenic plants against drought stress.
ACKNOWLEDGMENT
The authors are thankful to Prof. C. P. Malik, Prof. Reto Strasser and Prof. B. Robert for constant blessing and academic encouragement.
REFERENCES
- Araus JL, Slafer GA, Reynolds MP, and Royo C. Plant bree-dind and drought in C3 cereals: what should we breed for? Ann Bot, 2002; 89: 925- 940.
- Molnár I, Gáspár L, Sárvári É, Dulai S, Hoffmann B, Molnár-Láng M, and Galiba G. Physiological and morphological responses to water stress is Aegilops biuncia-lis and Triticum aestivum genotypes with differing tolerance to drought. Funct Plant Biol, 2004; 31: 1149-1159.
- Molnár I, Dulai S, Csernák Á, Prónay J, and Molnár-Láng M. Photosynthetic responses to drought stress in different Aegilops species. Acta Biol Szeged, 2005; 49(1-2): 141-142.
- Bohnert HJ, Nelson DE. and Jensen RG. Adaptations to environmental stresses. Plant Cell, 1995; 7: 1099-1111.
- Jaworski E. Nitrate reductase assay in intact plant tissues. Biochem Biophys Res Commun, 1971; 43: 1247-1279.
- Racusen D and Foote M. Protein synthesis in dark grown bean leaves. Can J Bot, 1965; 43: 817-824.
- Zink MW, and Veliky IA. Acid phosphatase of Ipomoea spp. cultured in vitro. 1. Influence of pH and inorganic phosphate on the formation of phosphatases. Can J Bot, 1979; 57: 739-753.
- Bernfeld P. Amylases ? and ?. Methods in Enzymol, 1955; 1:149-158.
- Harris GP and Jeffcoat B. Effects of temperature on the distribution of 14C-labelled assimilate in the flowering shoot of carnation. Ann Bot, 1974; 38: 77-83.
- Bray EA. Plant responses to water deficit. Trends Plant Sci, 1997; 2: 48-54.
- Tabaeizadeh Z. Drought induced responses in plant cells. Int Rev Cyto, 1998; 182:193-247.
- Grumet R, and Hanson AD. Genetic evidence for an osmo-regulatory function of glycine betanin accumulation in bar-ley. Aus J Plant Physiol, 1986; 13: 353-364.
- Garg BK, Kathju S, Lahiri AN, and Vyas SP. Drought resistance in pearl millet. Biologia Plantarum, 1981; 23: 182-185.
- Saxena HK, Yadav RS, Parihar SKS, Singh HB, and Singh GS. Susceptibility and recovery potential of rice genotypes to drought at different growth stages. Ind J Plant Physiol, 1996; 1: 198-202.
- Morilla CA, Boyer JS, and Hageman RL. Nitrate reductase activity and polyribosomal content of corn (Zea mays L.) having low leaf water potentials. Plant Physiol, 1973; 51: 817-824.
- Rajagopal V, Balasubramanian V, and Sinha K. Diurnal fluctuations in relative water content, nitrate reductase and proline content in water stressed and non-stressed wheat. Physiol Plant, 1977; 40: 69-71.
- Teare ID, Manam R, and Kanemasu ET. Diurnal and seasonal trends in nitrate reductase activity in field grown Sorghum plants. J Agron, 1974; 66: 733-736.
- Alekseeva AY, and Ramazanova LK. Catalytic functions of chloroplast peroxidase under dehydration conditions. Izv. Akad. Ser. Biol. Navk. (SSR), 1973; 6: 903-905.
- Chiung-Yueh S, and Ching-Hvei K. Induction of acid phosphatase in detached rice leaves under stress conditions. Bot Bull Head Sin, 1998; 39: 29-32.
- Sharma AD, Thakur M, Rana M, and Singh K. Effect of plant growth hormones and abiotic stresses on germination, growth and phosphatase activities in Sorghum bicolor (L.) Moench seeds. African J Biotech, 2004; 3(6): 308-312.
- Olmos E, and Hellin E. Cytochemical localization of ATpase plasma membrane and acid phosphatase by cerium based in a salt-adapted cell line of Pisum sativum. J Exp Bot, 1997; 48: 1529-1535.
- Garg BK, Vyas SP, Kathju S, and Lahiri AN. Influence of water deficit stress at various growth stages on some enzymes of nitrogen metabolism and yield in cluster bean genotypes. Ind J Plant Physiol, 1998; 3(3): 214-218.
- Thind SK, and Malik CP. Role of acid invertase and glycosi-dases in growth of wheat seedlings subjected variable water stress levels. Ind J Exp Biol, 1989; 27: 279-289.
| How to cite this article: Soni V, Swarnkar PL: Drought Induced Biochemical Changes in Commiphora wightii. Int. J. Life. Sci. Scienti. Res., 2017; 3(4):1247-1249. DOI:10.21276/ijlssr.2017.3.4.24 Source of Financial Support: Nil, Conflict of interest: Nil |