Int. J. Life. Sci. Scienti. Res., 4(2): 1698-1702, March 2018
Association
of Serum CRP level with Lung Cancer and Healthy Control of North Indian
Population
Priyanka
Gaur1*, Sarika Pandey2, Sandeep
Bhattacharya1, Surya Kant2, R.A.S. Kushwaha2,
Rajiv Garg2, Mohammad Kaleem3, Abhishek
Dubey2
1Department
of Physiology, King George’s Medical University, UP, Lucknow-226010,
Uttar Pradesh, India
2Department
of Respiratory Medicine, King George’s Medical University, UP, Lucknow-226010,
Uttar Pradesh, India
3Department of Biochemistry, King George’s Medical
University, Lucknow- 226010, Uttar Pradesh, India
*Address
for Correspondence:
Priyanka Gaur, Ph.D. Scholar, Department of
Physiology, King George’s Medical University, Lucknow- 226010, Uttar Pradesh,
India
ABSTRACT-
Background: Lung cancer is the major cause of
cancer-related mortality worldwide. Chronic inflammation of the airway plays an
important role in the alternations of bronchial epithelium and lung
microenvironment, therefore provoking the pulmonary carcinogenesis and
progression of lung cancer. The results may suggest that high inflammation
level can be associated with the higher risk of lung cancer. CRP is an
acute-phase protein produced in the liver in response to elevated cytokine
levels after an inflammatory stimulus. C-reactive protein (CRP) a systemic
marker of chronic inflammation is associated with increased lung cancer risk.
Material
and Methodology: This case-control study
was conducted on 40 lung cancer patients and 30 healthy controls. CRP level was
measured in serum by ELISA kits.
Results: Elevated
serum CRP level was found in lung cancer patients as comparison to healthy
controls. This study shows significant association between the serum CRP level
of lung cancer patients and healthy controls (p<0.0001) and also showed
significant association between smoker, ex-smoker and non-smokers lung cancer
patients as well as in healthy controls (p<0.0001).
Conclusion: Higher
CRP levels were found in lung cancer patients as compared to healthy controls.
The higher CRP level was also observed in Smoker, Ex-smoker as compared to
non-smoker in lung cancer patients and healthy control.
Keywords: Lung
Cancer, CRP, Inflammatory Stimulus, Cardiovascular disease, Biomarker
INTRODUCTION - Lung
cancer is the major cause of cancer-related mortality in both men and women
worldwide [1]. Chronic inflammation in airway plays an
important role in the alternations of bronchial epithelium and lung
microenvironment provoking the pulmonary carcinogenesis and progression of lung
cancer. The results may suggest that high inflammation level can be associated
with the higher risk of lung cancer. It is known that proinflammatory cytokines
such as interleukin 1, interleukin 2, tumor necrosis factor alpha
and tumor growth factor are able to stimulate the production of
C-reactive protein (CRP) as well as influence survival, growth, mutation,
proliferation, differentiation, and migration of tumor cells [2]. C-reactive
protein (CRP) a systemic marker of chronic inflammation increases during the
host response to tissue injuries such as infection, trauma, myocardial
infarction and surgery [3]. Serum CRP levels are associated
with the risk of cardiovascular disease, colon cancer and elevated levels of
CRP have been reported as a risk factor for the development of colon cancer
also [4,5]. CRP is an acute-phase protein produced in the liver
in response to elevated cytokine levels after an inflammatory stimulus [6].
It has been found that acute-phase response is also seen in a variety of diseases
such as cardiovascular disease, diabetes, systemic inflammatory diseases, some
autoimmune disorders and cancer [7,8]. CRP levels have also
been used to predict cancer risk, detect cancer recurrence and determine
prognosis [9-11]. Elevated preoperative serum CRP has been
identified to be a significant prognostic factor in patients with colorectal,
oesophageal and hepatic carcinoma. Several studies have shown that NSCLC
Patients with elevated preoperative serum CRP levels has worse survival than
those patients with undetectable levels of CRP [12-14]. It is
well known that chronic inflammation is associated with lung carcinogenesis.
C-reactive protein (CRP) a systemic marker of chronic inflammation is
associated with increased lung cancer risk. Elevated levels of C-reactive
protein (CRP) have been associated with increased lung cancer risk in several
retrospectives and a few prospective studies [15-19]. It can
serve as a good biomarker as measuring levels at baseline will be helpful in
assessing severity and determining the progression of diseases like COPD and
lung cancer. Measuring CRP levels will also be helpful in determining the
efficacy of treatment [20,21]. This study aims to determine the
serum CRP level in lung cancer and healthy control and its association with the
smoking status.
MATERIAL AND
METHODS- This study was conducted at the Department
of Respiratory Medicine, King George’s Medical University, Lucknow, India.
This study was approved by the ethics committee of the corresponding
institution and participants gave their written informed consent. A total
of 40 histopathologicaly confirmed lung
cancer patients were enrolled in this study after excluding those having other
disorders such as COPD, asthma, tuberculosis, interstitial lung
disease, and 30 healthy controls without having the past history of any chronic
or acute disease for last one month were also enrolled to compare the serum CRP
levels of both the groups. Peripheral blood samples of lung cancer
patients and controls were collected. The blood sample was centrifuged for the
separation of serum at stored at -80 0C until analysis. Serum
CRP level of lung cancer patients and healthy controls was determined by the
ELISA method according to manufacture’s instruction.
Statistical
analysis- Data were analyzed using Graph Pad Prism
version 5 (Graph Pad software Inc.; La, Jolla, CA, USA). All demographic and
clinical data were expressed as mean±standarderror
of the mean (SEM) and percentage. The chi-square test was used for categorical
data and groups were compared by unpaired t-test or one-way analysis of
variance (ANOVA), p < 0.05 was considered significant.
RESULTS- The
demographic and clinical characteristics of lung cancer patients and controls
are shown in Table 1. The mean age of the lung cancer patients and control
group were not showing significant different (p=0.45). Out of 40
lung cancer patients 33(82.5%) was male and 7(17.5%) were female. The study
also comprises 24(80%) healthy control male and 6(20%) healthy control female.
This study comprises 19(47.5%) smokers, 8(20%) Ex-smoker and 13(32.5%)
Non-smoker Lung cancer while 9(30%) smoker, 5(16.7%) ex-smoker and 16(53.3%)
non-smoker control. A non-significant difference was found in smoking
history of lung cancer patients and control (p=0.215). It has been observed
that the weight and BMI were lower in lung cancer patients as compared to
controls and this difference is statistically significant (p<0.0001). In the
present study 19(47.5%) lung cancer patients were of adenocarcinoma and 17(42.5%)
were squamous cell carcinoma. Majority of lung cancer patients
37(92.2%) were stage iii/iv. Serum CRP level was elevated in lung cancer
patients as compared to control (Fig. 1). In this study, the significant
association was observed in the serum CRP level (P< 0.0001) in lung cancer
patient and healthy control. Levels of serum CRP between smokers, Ex-smoker and
Non- smoker lung cancer patients and Control were also compared. The higher CRP
level was observed in smoker, Ex-smoker as compared to non-smoker both in lung
cancer patients and control (Fig. 2). The present study indicates that the
significant association was found in serum CRP level in Smoker, Ex-smoker and
Non-smoker in lung cancer and control (p< 0.0001).
Table 1: Clinical
characteristics of Lung Cancer Patients and Healthy Control
|
Parameters |
Lung Cancer (N=40) |
Controls (N=30) |
P value |
|
Age |
55.73±1.82 |
53.93±1.82 |
0.449 |
|
Sex Male Female |
33(82.5%) 7(17.5%) |
24(80%) 6(20%) |
0.771 |
|
Height |
159.9 ±1.28 |
158.3±1.38 |
0.533 |
|
Weight |
47.58±1.25 |
55.03±1.70 |
< 0.0001* |
|
BMI |
18.67±0.41 |
22.06±0.74 |
< 0.0001* |
|
Smoking History Smoker Ex- Smoker Non Smoker |
19(47.5%) 8(20%) 13(37.5%) |
9(30%) 5(16.7%) 16(53.3%) |
0.2154 |
|
Histology Adenocarcinoma Squamous Cell Carcinoma Small Cell Carcinoma NSCC |
19(47.5%) 17(42.5%) 1(2.5%) 3(7.5%) |
- |
- |
|
Stage I/II III/IV |
3(7.5%) 37(92.3%) |
- |
- |
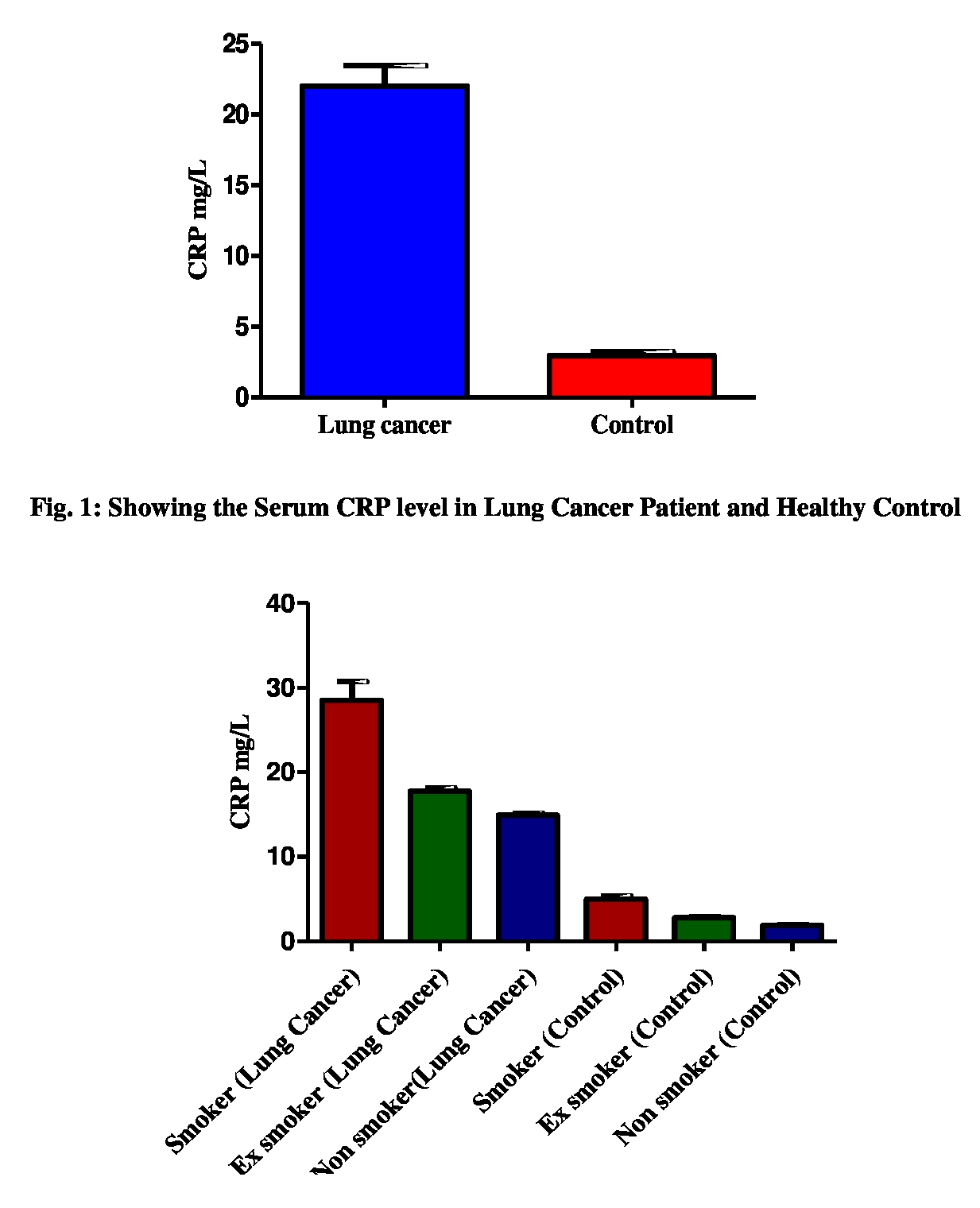
Fig.
2: Showing the Serum CRP level in smoker, Ex-smoker and Non-smoker Lung Cancer
patient and healthy control
DISCUSSION- CRP
was discovered in 1930, which is a representative acute-phase reactant whose
level was rapidly increased in response to most of the inflammation [22].
It is considered as one of the most widely used systemic inflammatory
markers in vivo condition [23]. CRP was
reported to be an informative biomarker, which reflects disease progression as
well as the efficacy of therapeutic intervention[24]. Serum CRP
levels begin to increase within 4-6 h after the onset
of inflammation and become at peak concentration at 36-50
h. After inflammation resolution serum levels decrease with a half-life of
less than 12 h [25]. As tumor growth can cause
tissue inflammation around the tumor and hence plasma levels of CRP
was increased. The mechanism by which cancer occurs along with increased CRP
level is widely known. It has been shown by the previous studies that CRP level
was elevated among former smoker and was associated with increased lung cancer
risk even among ex-smoker, who had quit smoking for up to 15 years. It has been
also found that high CRP levels among current smokers in relation to the amount
smoked, which support the notion of a role of inflammatory pathways in
tobacco-related lung cancer [15,17]. It has been shown from the
previous studies that the serum CRP level was highly elevated in lung cancer
patients when compared with healthy control [6,26]. Evidences
have indicated that cigarette smoke by itself can also induce pulmonary
inflammation [27]. Elevated CRP values were also detected in
NSCLC patients with larger tumor sizes, therefore being both an
important staging factor and prognostic factor [12]. Elevated
CRP has also been associated with increased weight loss, reduced performance
status, increased fatigue and decreased survival [28]. The
present study shows that the serum CRP level was higher in lung cancer patients
in comparison to healthy control and also the elevated level of CRP was found
in smokers as compared to Ex-smoker and Non-smoker in lung cancer patient and
healthy control. The significant association between the serum CRP level was
found in lung cancer patient as compared to healthy control (p<0.0001) and
also significant association was found in smoker, Ex-smoker and Non-smoker lung
cancer patient when compared with healthy control (p<0.0001). The
circulating CRP levels can be used as a useful prognostic predictor for
survival in lung cancer. Researchers focused on extending the clinical use of
circulating CRP to the prediction of cancer.
CONCLUSIONS- Significant
association of serum CRP level between the lung cancer patients and healthy
controls were found and also significant association between smoker, ex-smoker
and non-smoker lung cancer patients and healthy controls were found in this
study. The present study concluded that serum CRP level was higher in the lung
cancer patients when compared to healthy subjects. The elevated serum CRP level
was also found in smoker when compared with ex-smoker and non-smoker in lung
cancer patients and healthy controls. Serum CRP measurements are simple, rapid,
cost-effective. Smoking Cessation in patients
only reduces, it does not eliminate the risk of lung cancer because
inflammation persists even after smoking cessation. Therefore Smoking Cessation
along with CRP lowering agents may have promising roles for the prevention and
therapy of lung cancer.
ACKNOWLEDGEMENTS- We
are greatly thankful to Department of Physiology for providing necessary
facilities for carrying out this study. We are also appreciating the patients
and the healthy volunteers, who were participating in this study.
REFERENCES
1.
Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics. CA Cancer J Clin, 2009; 59(4):225-49.
2.
Coussens LM, Werb Z.
Inflammation and cancer. Nature, 2002; 420:860-7.
3.
Gabay C, Kushner I. Acute-phase proteins
and other systemic responses to inflammation. N Engl J
Med,1999; 340:448–454.
4.
Ridker PM, Buring JE,
Shih J, Matias M, Hennekens CH
Prospective study of C-reactive protein and the risk of future cardiovascular
events among apparently healthy women. Circulation,1998; 98:731–733.
5.
Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, et al. C-reactive protein and colorectal
cancer risk: a systematic review of prospective studies. Int J Cancer, 2008; 123:1133–1140.
6.
Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest, 2003; 111:1805-12.
7.
Bolayirly M, Turna H, Orhanoglu T, Ozaras R, Ilhan M, Ozguroglu M.
C-reactive protein as an acute phase protein in cancer patients. Med Oncol, 2007; 24:338-44.
8.
Sabatine MS, Morrow DA, Jablonski KA, Rice MM, Warnica JW, Domanski MJ, et al. Prognostic significance of
the Centers for Disease Control/American
Heart Association high-sensitivity C-reactive protein cut points for
cardiovascular and other outcomes in patients with stable coronary artery
disease. Circulation, 2007; 115:1528-36.
9.
Mahmoud FA, Rivera NI. The role of
C-reactive protein as a prognostic indicator in advanced cancer. Curr Oncol Rep, 2002;
4:250-5.
10.
Wilop S, Crysandt M, Bendel M, Mahnken AH, Osieka R, Jost E.
Correlation of C-reactive protein with survival and radiographic response to
first-line platinum-based chemotherapy in advanced non-small cell lung
cancer. Onkologie, 2008;
31:665-70.
11.
Chiu HM, Lin JT, Chen TH, Lee YC, Chiu YH, Liang JT, et al. Elevation of
C-reactive protein level is associated with synchronous and advanced
colorectal neoplasms in men. Am J Gastroenterol, 2008; 103:2317-25.
12.
Koch A, Fohlin H, Sorenson S.
Prognostic significance of C-reactive protein and smoking in patients with
advanced non-small cell lung cancer treated with first-line palliative
chemotherapy. J Thorac Oncol,
2009; 4:326-32.
13.
lan S, zhiming L,
shun L. Clinical significance of C-reactive protein in patients with stage
I nonsmall cell lung cancer. Chin J Oncol, 2011; 33:442-46.
14.
Hara M, Yonei A, Ayabe T, Tomita M, Nakamura K, Onitsuka T. Postoperative serum C-reactive protein
levels in non-small cell lung cancer patients. Ann Thorac Cardiovasc Surg, 2010;
16:85-90.
15.
Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-reactive protein is
associated with incident cancer and survival in patients with cancer. J Clin Oncol, 2009;
27:2217–2224.
16.
Chaturvedi AK, Caporaso NE, Katki HA, et al. C-reactive protein and risk of lung
cancer. J Clin Oncol,
2010; 28:2719–2726.
17.
Heikkila K, Ebrahim S, Lawlor DA. A systematic review of the association
between circulating concentrations of C reactive protein and cancer. J Epidemiol Community Health, 2007;61:824–833.
18.
Siemes C, Visser LE, Coebergh JW, et al. C-reactive protein levels,
variation in the C-reactive protein gene, and cancer risk: the Rotterdam Study.
J Clin Oncol,
2006; 24:5216–5222.
19.
Trichopoulos D, Psaltopoulou T,
Orfanos P, et al. Plasma C-reactive protein and risk of cancer: a prospective
study from Greece. Cancer Epidemiol Biomarkers
Prev., 2006; 15(2):381–384.
20.
Il’yasova D, Colbert LH, Harris TB, et al:
Circulating levels of inflammatory markers
and cancer risk in the health aging and body composition cohort. Cancer Epidemiol Biomarkers Prev,
2005; 14:2413-2418.
21.
Pandey S, Garg R,
Kant S, Gaur P, Singh S, Singh P. C-Reactive Protein as a Biomarker in Chronic
Obstructive Pulmonary Disease Patients: A Mini Review. Int. J. Life. Sci. Scienti. Res., 2018; 4(1):1534-1535.
22.
Shiels MS, Pfeiffer RM, Hildesheim A,
Engels EA, Kemp TJ, Park JH, Katki HA, Koshiol J, Shelton G, Caporaso NE,
Pinto LA, Chaturvedi AK. Circulating
inflammation markers and prospective risk for lung cancer. J Natl Cancer Inst, 2013; 105:1871-80.
23.
Tillett WS, Francis T. Serological
Reactions In Pneumonia with a Non-Protein Somatic Fraction Of Pneumococcus. J Exp Med, 1930; 52:561-71.
24.
Hotta K, Sho M,
Fujimoto K, Shimada K, Yamato I, Anai S, Konishi N, Hirao Y, Nonomura K, Nakajima Y. Prognostic significance of
CD45RO+ memory T cells in renal cell carcinoma. Br J Cancer, 2011; 105:1191-6.
25.
Masago K, Fujita S, Togashi Y, Kim YH, Hatachi Y, Fukuhara A, Nagai H, Irisa K, Sakamori Y, Ocuda C,
Mio T, Mishima M. significance of pretreatment C-reactive protein in patients with
advanced nonsquamous, nonsmall cell
lung cancer who received gefitinib. Oncology,
2010; 79:355-6.
26.
Chung HW, Kim JW, Lee JH, Song Sy, Chung
JB, KwonOH, et al. Comparison of the validity of
three biomarkers for gastric cancer screening: carcinoembryonic antigen, pepsinogens, and high sensitive C-reactive protein. J Clin Gastroenterol, 2009;
43:19-26.
27.
Engels EA. Inflammation in the development of lung cancer:
Epidemiological evidence. Expert Rev Anticancer Ther,
2008; 8:605–615.
28.
Scott HR, McMillan DC, Forrest LM, Brown DJ, McArdle CS,
Milroy R. The systemic inflammatory response, weight loss, performance status
and survival in patients with inoperable non-small cell lung cancer.
Br J Cancer, 2002; 87:264-7.