RESULTS- Phytochemical
analysis of the leaves of Spinacia
oleracea L. had most of the important phytoconstituents like Alkaloids,
Reducing sugar, Flavonoids, Glycosides, Cardiac glycosides, Tannins, Saponin,
Protein, Amino acid, Terpenoids, and steroids. Alkaloids are a class of
nitrogenous organic compounds of plant origin which have diverse
and important physiological effects on humans and other animals. All the
tests for alkaloids i.e. Mayer’s test, Wagner’ test, Hager’s test shows positive
in all the extract. Mayer’s test shows negative with aqueous extraction.
Carbohydrate is absent by all the tests used for aquous, quath and methanolic
extraction. While reducing sugar was detected in all the extracts (except
methanolic extraction by Fehling’s test). Flavonoids are hydroxylated
polyphenolic compounds that carry out important functions in plants, including
attracting pollinating insects; combating environmental stresses, such as
microbial infection; and regulating cell growth. Six major subclasses of
flavonoids, namely anthocyanidins, flavan-3-ols, flavonols, flavanones,
flavones, and isoflavones; flavonols are the most widespread in the human diet.
Aqueous and quath extraction shows presence of flavonoids by all the tests
used. Methanolic extraction was shown presence of flavonoids only with lead
acetate test. Tests for glycosides shows variable results and varies from test
to test as well as extraction procedure and solvents. Cardiac glycosides are
class of organic compounds that increase the output force of the heart and
decrease its rate of contraction by acting on the cellular Na-K ATPase pump.
Cardiac glycosides were present only in quath but absent in aqueous and
methanolic extraction. Phenolic compounds are any compounds derived from the
phenol group and contribute to the colour, structure, astringency, etc. Tannins
are large molecular weight compounds resulting from polymerization reaction of
smaller phenolic compounds. Tannins and phenolics are mostly positive with
tests we applied. Saponin is present in all the extracts. Amino acids and
proteins are absent in quath extraction. Ninhydrin tests show positive while
Biuret test shows negative results with aquas and methanolic extraction.
Terpenoids are present in aqueous and methanolic extraction. Further, steroids
are present in all the extracts. Further, the antioxidant activities were
observed in all the extract i.e. aqueous, quath, and methanolic extraction.
Thin layer chromatography- The
thin layer chromatography of sample shows different spots for methanolic, quath
and auous extraction of Spinacia oleracea. In the methanolic and aqueous
(10 gm) extraction of palak shows 6 spots having Rf 0.33, 0.44,
0.55, 0.66, 0.77, 0.83 and 0.16, 0.33, 0.35, 0.44, 0.55, 0.66 respectively. But
in 5 gm aquous extraction only 4 spots revealed having Rf 0.38,
0.44, 0.55, 0.66 respectively (Fig. 1).
Table 1: Phytochemicals analysis of aqueous, quath
and methanolic extracts of Spinacia
oleracea L. leaves
|
S. No. |
Phytochemical Test |
Palak Aqueous |
|||
|
Quath +ve +ve +ve |
5
gm 10 gm |
Methanolic |
|||
|
1. |
Test for alkaloids (a) Mayer’s test (b) Wagner’ test (c) Hager’s test |
-ve +ve +ve |
-ve +ve +ve |
+ve +ve +ve |
|
|
2. |
Test for carbohydrate (a) Molisch test (b) Barfoed’s test |
-ve -ve |
-ve -ve |
-ve -ve |
-ve -ve |
|
3. |
TEST FOR REDUCING SUGAR (a) Fehling’s test (b) Benedict’s test |
+ve +ve |
+ve +ve |
+ve +ve |
-ve +ve |
|
4. |
TEST FOR FLAVONOIDS (a) Alkaline reagent (b) Lead acetate (c) Ammonia test |
+ve +ve +ve |
+ve +ve +ve |
+ve +ve +ve |
-ve +ve -ve |
|
5. |
TEST OF GLYCOSIDES (a) Borntrager test (b) Legal’s test (c) 10% NaOH test |
+ve -ve +ve |
-ve -ve +ve |
-ve -ve +ve |
+ve -ve +ve |
|
6. |
TEST OF CARDIAC GLYCOSIDES (a) Keller killani test |
+ve |
-ve |
-ve |
-ve |
|
7. |
TANNIN AND PHENOLIC TEST (a) Ferric chloride test (b) Lead acetate test (c) Dilute iodine test (d) Ferric chloride 10% (e) Hydrolysable tannin |
+ve +ve +ve -ve -ve |
-ve +ve +ve -ve +ve |
-ve +ve +ve -ve +ve |
+ve +ve +ve +ve +ve |
|
8. |
TEST FOR SAPONIN (a) Saponin test |
+ve |
+ve |
+ve |
+ve |
|
9. |
AMINO AND PROTEIN (a) Ninhydrin test (b) Biuret test |
-ve -ve |
+ve -ve |
+ve -ve |
+ve -ve |
|
10. |
TERPENOIDS & STEROID
(a) Test for terpenoids (b) Test
for steroid |
-ve +ve |
+ve +ve |
+ve +ve |
+ve +ve |
“+” = Positive (Present); “-” =
Negative (Absent)
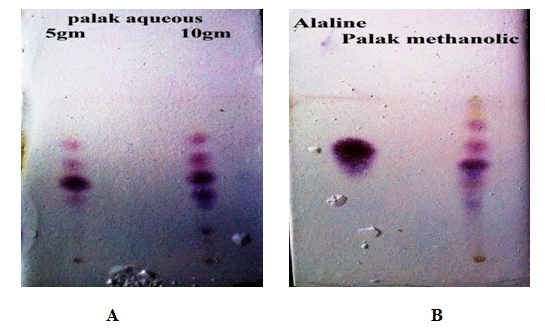
Fig. 1: TLC-Plate showing different
solvent extract of leaves of Spinacia
oleracea L.
(A.
5 gm & 10 gm Aqueous Extract, B.
Methanolic Extract)
DISCUSSION-
The
preliminary phytochemical analysis of Spinacia oleracea revealed that
different active constituent present in different extracts such as
carbohydrates, proteins, amino acids, fat, oils, steroids, terpenoids,
glycosides, alkaloids, tannins and other phenolic compounds [16] but
in our study alkaloids, reducing sugar, flavonoids, glycosides, tannins &
phenolic compounds, saponin, amino acids, terpenoids and steroids are present
because we applied more than one methods for phytochemical screening. Medicinal
values of plants have assumed an important dimension in the past few decades.
Plants produce a very diverse group of secondary metabolites with antioxidant
potential. Antioxidants block the action of free radicals which have been
implicated in the pathogenesis of many diseases and in the aging process [17-19]. An important role is
being played by free radicals in governing the various biological processes
which are necessary for the body. They have their role in implicating
cell-signaling mechanism occurring in our body.
As
prevention is a more effective strategy than treatment for chronic diseases, a
constant supply of phytochemical containing plants with desirable health
benefits beyond basic nutrition is essential in reducing the risk of diseases
in humans. The importance of these phytochemicals is their presumed ability to
inhibit carcinogenesis. They play a variety of roles such as antioxidants,
inhibitor of tumor growth, anti-mutagens, enzyme modulators, chemical
inactivators, and free radical scavengers [20]. Terpenoids reduce
complications associated with diabetes and lowers the sugar level in blood [21].
Further, terpenoids have been found to be very useful in anti-aging and overall
beauty enhancement. Hawkins and Ehrlich [21] reported that
terpenoids have capacity to improve lung function in respiratory treatment.
Cardiac glycosides showed to aid in treatment of congestive heart failure and
cardiac arrhythmia.
Free radicals are continuously produced
in the human body, as they are essential for energy supply, detoxification,
chemical signaling and immune function but they
are also involved in various diseases such as diabetes [22], rheumatoid arthritis [23-24],
high blood pressure [25], urinary tract disorders [26],
bronchial asthma [27-28] and non-healing
wounds [29]. Free radicals can initiate the oxidation
of bio molecules, such as protein, lipid, amino acids and DNA which will lead
to cell injury and can induce numerous diseases [30]. An imbalance between antioxidants and reactive oxygen
species results in oxidative stress, leading to cellular damage and oxidative
stress is the main cause of several diseases: cancer, cataracts, age related
diseases and Parkinson’s disease. Antioxidants reduce the oxidative stress in
cells and are therefore useful in the treatment of many human diseases,
including cancer, cardiovascular diseases and inflammatory diseases. This
activity is due to the ability of antioxidants to reduce oxidative stress by
neutralizing or scavenging of reactive species by hydrogen donation [31-32].
The natural drugs are always a better
substitute for synthetic drugs. Thus numerous drugs have entered the I.P
through ethno botany and traditional medicine. The medicinal value of a plant
lies on bioactive phytochemical constituents that produce a definite
physiological action on the human body. These phytoconstituents work with
nutrients and fibers to form an integrated part of defense system against
various diseases and stress conditions. The most important of these bioactive
constituents of plants are tannins, flavonoids, carbohydrates, glycosides,
steroids, terpenoids, lignin’s, and fats [33]. Phenolic compounds in
general and flavonoids in particular have the ability to provide protection
against oxidative stress. Thus in this study, the presence of flavonoids and
phenolic compounds in the extract could be considered responsible for
conferring antioxidant ability.
CONCLUSIONS- Spinacia oleracea L. is a
leafy vegetable that belongs to the goosefoot family. It has various
pharmacological activities such as anti-oxidant, antiproliferative,
anti-infammatory, antihistaminic, CNS depressant, protection against gamma
radiation, hepatoprotective, etc. Phytochemical
investigation of Spinacia oleracea L. were shown the presence of
Alkaloids, Reducing sugar, Flavonoids, Glycosides, Cardiac glycosides, Tannins,
Saponin, Protein, Amino acid, Terpenoids and steroids etc. There was no effect
of concentration on the phytochemical contituents. Mostly results are same in
aqueous and quath extract so we concluded that there is no need to dry the
green leaves. Result depends upon the solvent as well as the method of test we
apply. Further, we observe the antioxidant activities in the extracts. Thus, Spinacia oleracea merits further
phytochemical, pharmacological and clinical investigations for development of
an effective natural remedy to provide therapeutically effective lead compounds
or extracts. This vegetable can be used as a therapeutic and curative
medicine for many oxidative stress- induced diseases.
REFERENCES
1.
Edoga HO, Okwu DE, Mbaebie BO.
Phytochemicals constituents of some Nigerian medicinal plants. Afr J
Biotechnol, 2005; 4:685-688.
2.
Mann J. Secondary Metabolism. Oxford
University press, London 1978; 154.
3.
Alta MVA, Adeogun OA. Nutrient
components of some tropical leafy vegetables. J Food Chem, 1995; 53:375 - 379.
4.
Williamson G, Dupont MS, HeaneyRK,
RogerG, Rhodes MJ. Induction of glutathione S transferase activity in hepG2
cells by extracts of fruits and vegetables. Food Chem, 1997;2:157-160.
5.
Liu RH. Potential synergy of
phytochemicals in cancer prevention: mechanism of action. J. Nutrition, 2004;
134: 34795-34855.
6.
Oduse Kayode A, Idowu Micheal A,
Adegbite Adefolawe A. IOSR Journal of Environmental Science, Toxicology and
Food Technology, 2012; 1: 22-26.
7.
Vasthi kennedi Evenjelene. Evaluation of
free radical scavenging activity and biological properties of Spinacia oleracea L. International
Journal of Engineering science and Technology, 2011; 3:25-29.
8.
Rukmana R. Bertanam Bayam dan
pengelolaan pascapanen. Kanisius. Yogyakarta, 1994.
9.
Boivin D, Lamy S, Lord-Dufour S, Jackson
J, Beaulieu E, Cote M, Moghrabi A, Barrette S, Ginras D, Beliveau R. Food
Chemistry, 2009;112: 374–380.
10. Hait-Darshan
R, Grossman S, Bergman M, Deutsch M, Zurgil N. Food Research International,
2009; 42:246–253.
11. Onyeka EU, Nwambekwe IO. Phytochemical profile of
some green leafy vegetables in South East, Nigeria. Nigerian
Food Journal, 2007; 25:
67-76.
12. Miller
HE, Rigelhof F, Marquart L, Prakash A, and Kanter M. Whole-grain products and antioxidants,. Cereal Foods World, 2000;
45:59-63.
13. Miller, H.E., Rigelhof, F., Marquart, L., Prakash, A., and Kanter, M.
Antioxidant content of whole grain breakfast cereals, fruits and vegetables. J.
Am. Coll. Nutr, 2000; 19:312S-319S.
14. Dung N. X. and Dinh, T., 2005,
Extraction and Distillation of essential Oils, Processing, Analysis and
Application of Essential Oils, 1st Edition, Har Krishan Bhalla & Sons Book
Company, p. 59
15. Singh
V and Kumar R. Study of Phytochemical
Analysis and Antioxidant Activity of Allium sativum of Bundelkhand
Region. Int. J. Life. Sci. Scienti.
Res., 2017; 3(6):1451-1458.
16. Prieto P, Pineda M,
Anguilar, M. Spectrophotometric quantitation of antioxidant capacity through
the formation of a Phosphomolybdenum Complex: Specific application to the
determination of Vitamin E. Anal. Biochem. 1999, 26 9, 337-341.
17. Aruoma
OI. Methodological considerations for characterizing potential antioxidant
actions of bioactive components in plant foods. Mutation Res. 2003; 523:9-20.
18. Dasgupta
N, De B. Antioxidant activity of Piper
betle L. Leaf extract in vitro.
Food Chem, 2004; 88:219-224.
19. Coruh
N, Celep AGS, Ozgokce F. Antioxidant properties of Prangos ferulacea (L) Lindl, Chaerophyllum
macropodum Boiss. And Heracleum
persicum Desf. From Apiaceae family used as food in Eastern Anatolia and
their inhibitory effects on glutathione-S-transferase. Food Chem 2007;
100:1237-1242.
20. Elias
K. Mibei, Nelson K. O. Ojijo, Simon M. Karanja, Johnson K. Kinyua.
Phytochemical and antioxidant analysis of methanolic extracts of four African
indigenous leafy vegetables. Annals. Food Science and Technology, 2012;
13:37-42.
21. Hawkins
EB, Ehrlich SD. Gotu Kola. University of Maryland Medical Center. Baltimore.
USA. 2006.
22. Ceriello A. Oxidative stress and
diabetes-associated complications. Endocr Pract, 2006; 12:60-62.
23. Nourmohammadi I, Athari-Nikazm S, Vafa
M, Bidari A, Jazayeri S, Hoshyarrad A. Effects of antioxidant supplementations
on oxidative stress in rheumatoid arthritis patients. J Biol Sci., 2010; 10:63-66.
24. Silva BN, Araújo ÍL, Queiroz PM, Duarte
AL, Burgos MG. Intake of antioxidants in patients with rheumatoid arthritis. Rev Assoc Med Bras. 2014; 60:555–559.
25. Baradaran A, Nasri H, Rafieian-Kopaei M.
Oxidative stress and hypertension: Possibility of hypertension therapy with
antioxidants. J Res Med Sci., 2014; 19:358–367.
26. Delshad M, Fesharakinia A, Eghbal S. The
role of oxidative stress in pediatric urinary tract infections: A systematic
review. Rev Clin Med. 2016; 3:43–47.
27.
Nadeem
A, Chhabra SK, Masood A, Raj HG. Increased oxidative stress and altered levels
of antioxidants in asthma. J Allergy Clin Immunol., 2003; 111:72–78.
28.
Picado
C, Deulofeu R, Lleonart R, Agustí M, Mullol J, Quinto L, et al. Dietary micronutrients/antioxidants and their relationship
with bronchial asthma severity. Allergy, 2001;
56:43-49.
29. Aggarwal S, Sardana S. Medicinal plants with wound healing and antioxidant activity: An update. Int J Pharm Innov., 2013; 3:30–40.
30. K.N.V. Rao, Bushra Tabassum, S. Raghu Babu, Alagara
Raja, David Banji. Eliminary Phytochemical
Screening of Spinacia Oleracea L.
World Journal of Pharmacy And Pharmaceutical Sciences, 2015; 4:532-551.
31. Hsu
C, Chen W, Weng Y, Tseng C. Chemical composition, physical properties, and
antioxidant activities of yam fluors as affected by different drying methods.
Food Chem, 2003; 83:85-92.
32. Erkan
N, Ayranci G, Ayranci E. Antioxidant activity of rosemary (Rosmarinus
officinalis) extract, Black seed (Nigella sativa) essential
oil , carnosic acid, rosmarinic acid and sesamol. Food Chem, 2008; 110:76-82.
33. Ergene
A, Guler P, Tan S, Mirici S, Hamzaoglu E, Duran A. Antimicrobial and antifungal
activity of Heracleum sphondylium
subsp. artivinense, African Journal of Biotechnology, 2006; 5: 1087–1089.