Int. J. Life. Sci. Scienti. Res.,
3(3):
1039-1046,
May 2017
Evaluation of Antagonist Activity
of Trichoderma Species Against Alternaria alternata Isolated from Populus deltoides
Kartik Uniyal*
and Y.P. Singh
Forest Pathology Division, Forest Research
Institute, Dehradun-248006
ABSTRACT- Populus deltoides
is the exotic species of poplar introduced in India in late 50s and has been
grown significantly in North– western states. It is one of the most important
commercial tree planted in agrosilvicutural system
adopted by farmers of the region. Meanwhile, it is prone to number of biotic
and abiotic agents, which affects the plantations and
thus depreciates its quality. Hence, to prevent the loss and manage the
diseases, apart from fungicidal use biocontrol
strategy has been adopted. In the present study fifteen isolates of Alternaria alternata has
been tested against Trichoderma harzianum and
Trichoderma viride. Both
the antagonists were at par in suppressing the fungal growth and did not
achieve the significant level of inhibition. T. harzianum could be shown as better biocontrol agent than the latter owing to the percent
growth inhibition shown by the isolates.
Keywords: Populus deltoides, Alternaria alternata, Biocontrol, Growth
suppression
Introduction- Poplars
are among the leading commercial tree species of the world in view of their
rapid growth and suitability for extensive range of products. They contribute significantly to
some national and regional wood markets [1] and also serve as a substantial
source of farm income in some countries [2]. Six
indigenous (P. alba, P. ciliata, P. euphratica, P. gamblei, P. glauca and P. suvolensis [3]; and three exotic (P. deltoides, P. nigra and their hybrid, P. x. eumericana [4] ; species
of poplars are reported in India. Poplars suffer from various diseases owing to
its monoculture plantations. Incidence of Alternaria alternata causing leaf spot was
predominant on clones of P. deltoides during 2010-2012 in the nurseries of WIMCO seedlings.
Disease problems have, therefore, posed the question regarding the overuse of
single clones and use of large monoclonal plantations [5].
Thus to manage the outbreak of disease, besides the use of fungicides
bio-control strategy is also a potential alternative. Biological control aims
at managing the plant pathogenic populations at natural levels. It is the
reduction of inoculums density of pathogens by one or more organisms,
accomplished either naturally or through manipulation of the environment, host
or antagonists [6]. This is an eco-friendly approach and best
alternative to chemical management. Among the fungal biocontrol
agents, Trichoderma is one of the most
commonly used organisms for the control of soil borne fungal pathogens and
considered as effective antagonist against plant pathogenic fungi [7-9].
MATERIALS AND
METHODS- Total 15 isolates of A. alternata obtained from different
commercial clones of P. deltoides (G48, WSL22, WSL39 and Udai)
were tested against two Trichoderma species, viz., T. harzianum and T. viride (antagonists) in 2012-13. The
cultures of antagonists were obtained from Forest Pathology Division,
Forest Pathology Division, DehraDun, India. In-vitro biological activity of antagonists on A. alternata was investigated on the potato dextrose agar
(PDA) using Dual Culture Method [10]. The experiment was conducted
in triplicates. The control plates were also maintained in which a colony of
test fungus was placed on one end of the Petri plate, while in experimental
plates a colony of test fungus was placed on one end and antagonist colony at
other end parallel to each other. The plates were incubated in BOD incubator at
27 ±1oC till
the test pathogen attains maximum radial growth in the control plates. Radial growth of A. alternata
isolates were recorded and percent inhibition was calculated using the
formula [11].
Percentage
inhibition (I) = (Control (C) – treatment (T) / Control) x100
I = C-T / C × 100
Data
was analyzed with the help of GENSTAT 5 Release 3.22. Two-way analysis was used
for biocontrol sensitivity data. Treatments means
were compared at 5 percent level of significance.
RESULTS AND DISCUSSION- The
two antagonist isolates (T. harzianum and T. viride)
showed different behaviour against the isolates of
test pathogen, A. alternata (Table1 & Fig.1.). Irrespective of
antagonists, maximum and significantly high antagonists’ efficiency was observed
against isolate number A51 (45.9%) which was at par with isolate No. A12
(44.5%), while significantly low value was registered for isolate No. A15
(24.4%). Both the Trichoderma
species expressed significantly different suppression of the growth of pathogen
(33.4 and 35.0% by T. viride and T. harzianum,
respectively) when pathogenic isolates were ignored.
On studying the interactions between
pathogen and antagonist (P x A), significantly high growth suppression was
achieved for isolate No. A12 (49.7%) by T.
viridae. Whereas, minimum and significantly less
growth inhibition was seen for isolate No. A25 (19.4%) by T. harzianum. Eight isolates, No. A7
(35.1%), A13 (39.4%), A16 (44.4%), A41 (44.7%), A47 (47.0%), A51 (48.6%), A52
(38.7%), A65 (38.0%) had maximum growth suppression by T. harzianum. While, remaining seven
isolates, No. A12, A15, A24, A25, A32, A40 and A64 (49.7%, 25.8%, 29.8%, 31.8%,
32.7%, 45.9% & 44.4%, respectively) were inhibited maximally by T. viride.
Table 1: Efficacy of Trichoderma species against A. alternata isolates
|
Isolate No. |
Antagonist/Growth
inhibition (%) |
Mean |
|
|
T. harzianum |
T. viridae |
|
|
|
A7 |
35.1 |
32.3 |
33.7 |
|
A12 |
39.2 |
49.7 |
44.5 |
|
A13 |
39.4 |
22.2 |
30.8 |
|
A15 |
23.1 |
25.8 |
24.4 |
|
A16 |
44.4 |
20.3 |
32.4 |
|
A24 |
24.4 |
29.8 |
27.1 |
|
A25 |
19.4 |
31.8 |
25.6 |
|
A32 |
24.3 |
32.7 |
28.5 |
|
A40 |
21.4 |
45.9 |
33.7 |
|
A41 |
44.7 |
40.2 |
42.4 |
|
A47 |
47.0 |
25.7 |
36.4 |
|
A51 |
48.6 |
43.1 |
45.9 |
|
A52 |
38.7 |
25.8 |
32.3 |
|
A64 |
37.1 |
44.4 |
40.7 |
|
A65 |
38.0 |
31.3 |
34.6 |
|
Mean |
35.0 |
33.4 |
|
|
|
Pathogen (P) |
Antagonist (A) |
Interactions
(P x A) |
|
SEM |
0.7 |
0.3 |
1.0 |
|
CD (5%) |
2.0 |
0.7 |
2.8 |
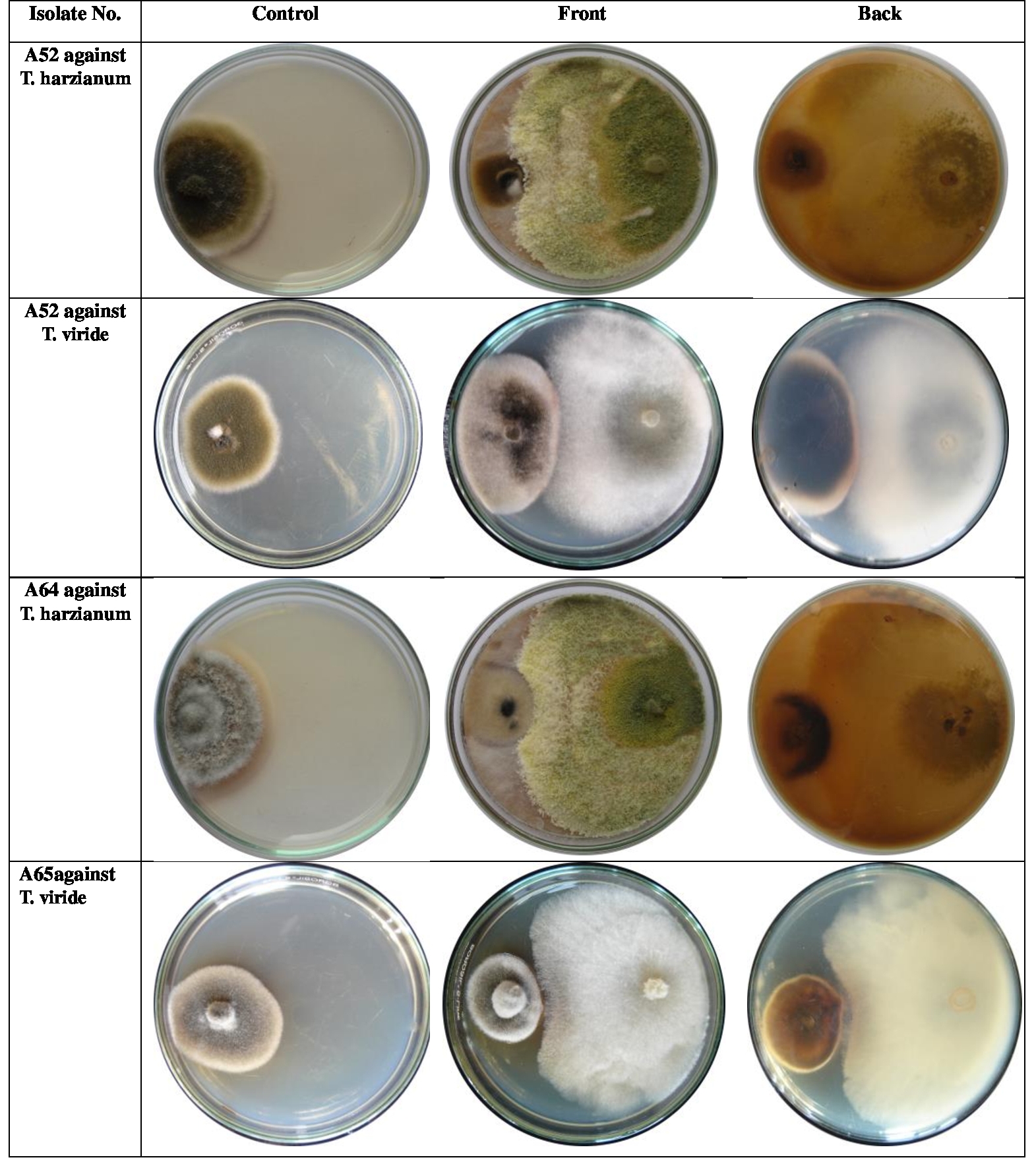
Fig. 1: Interactions between A. alternata isolates and Trichoderma species
T. harzianum is
an efficient biocontrol agent that is commercially
produced to prevent development of several soil and foliar pathogenic fungi [12-14].
Trichoderma strains are among the most
studied fungal biocontrol agents [15]. Different mechanisms have been suggested as
being responsible for their biocontrol activity,
which includes competition for space and nutrients, secretion of chitinolytic enzymes, mycoparasitism
and production of inhibitory compounds [16,17]. The diversity of
mechanism available to Trichoderma sp
for pathogen suppression through broad range of antifungal metabolites
production, mycoparasitism, competition with pathogen
of nutrient and occupation ofinfection court, induced
resistance [18]. In the present case, Trichoderma species expressed at
par mean growth suppression of the pathogen A.
alternata (around 30%). However, isolates
exhibited differential sensitivity to the antagonist, example, eight isolates
had maximum growth suppression by T. harzianum. While, remaining seven isolates were
inhibited maximally by T. viridae. T. harzianum could
be assigned as slightly better biocontrol agent than T. viride though
both the antagonists did not touch the magical mark of 50 percent. Therefore,
these strains of antagonist may not be recommended for the biological
management of A. alternata.
Contrary to the present observation, T. harzianum was reported to be effective biocontrol agent against A. alternata isolated from Capsicum frutescens
as their suppression range was around 70 percent [19]. While the
findings of [12, 20- 21] suggested that Trichodema
sp. were capable enough to inhibit the growth of Alternaria
species to a significant level.
CONCLUSION- The present investigation suggests that the
pathogen, Alternaria alternata
could not be efficiently suppressed by Trichoderma
species tested, which is contrary to the cited literature. It may be due to the
different ecological niche of the isolated pathogen and antagonists. Also, the
shift of sleeper pathogen, i.e. Alternaria alternata to the epidemic scale in poplar nurseries and
its virulence may be the possible reason that this potent biocontrol
agent fail to inhibit the pathogen growth in vitro. The study paves the way for
further testing of different Trichoderma spp. against
this pathogen to find out the efficient and better biocontrol
agent.
REFERENCE
1.
Viart
M. 1979. Silviculture of temperate and semi temperate
forests. In: A technical account of study tour made in India, October 8 to 15,
1979, pp:15.
2.
Prevosto
M. 1979. Growth and revenue of poplar grown in specialized stands subjected to
or not to thinning (in Italy). In: Technical consultation on fast growing
plantation broadleaved trees for Mediterranean and temperate zones, vol. 2,
Lisbon, Portugal, pp: 95- 114.
3.
Naithani HB, Chandra S, and Pal M. Indian
poplars with special reference to indigenous species. Indian Forester, 2001;
127 (2): 230-237.
4. Kaul
RN and Sharma KK. Status report on poplars. In: Proceedings of Workshop on
poplars at Haldwani. FRI and Colleges, Dehradun. 1982, pp:21-53.
5.
Stelzer
HE and Goldfarb B.. Implementing clonal forestry in south- eastern United States. Cand J For Res, 1997; 27: 442-446.
6.
Singh RS. Plant Disease. 9th ed.,
New Delhi; Oxford & IBH Publishing Co. Pvt. Ltd.: 2015: 700p.
7.
Chet I, Harman
GE, and Baker R. Trichoderma
hamatum: its hyphal interaction
with Rhizoctonia solani
andPythium spp. Micro Biol. 1981; 7: 29-38.
8.
Kumar RN and Mukerji KG. Integrated disease
management- future perspectives. In:
KGMukerji, BMathur,
BP Chamala and C Chitralekha
(Eds.), Advances in Botany. New
Delhi; APH Publishing Corporation: 1996,
pp: 335-347.
9.
Harma
GE. Myths and dogmas of biocontrol: changes in
perceptions derived from research on Trichoderma
harzianum T22. Plant Dis,
2000; 84: 377-393.
10. Dhingra
OD and Sinclair JB. Establishment of disease and testing for resistance. In: Basic Plant Pathology Methods,
2nd ed., Boca Raton; CRC Lewis Publishers: 1995, pp: 434.
11. Vincet
JH. Distortion of fungal hyphae in presence of
certain inhibitors. Nature, 1947;
159: 850.
12. Murtaza A, Shafique S, Anjum
T, and Shafique S.. In
vitro control of Alternaria citri using
antifungal potentials of Trichoderma species.
Afr J Biotechnol, 2012; 11(42): 9985-9992.
13. Hanada
RE, Pomella AWV, Soberanis
W, Loguercio LL, and Pereira JO. Biocontrol
potential of Trichoderma martiale against the black-pod disease (Phytophthora palmivora)
of cacao. Biol. Control, 2009; 50:143-149.
14. Nallathambi
P, Umamaheswari C, Thakore
BBL, and More TA. Post-harvest management of ber (Ziziphus mauritiana Lamk) fruit rot (Alternaria
alternata Fr. Keissler)
using Trichoderma species, fungicides
and their combinations. Crop Prot, 2009; 28: 225-232.
15. Vinale
F, Ghisalberti EL, Sivasithamparam
K, Marra R, Scala F, and Lorito M.
Secondary metabolites produced by Trichoderma
spp. and their role in the interaction of this fungus with plants and other
microorganism. Proceedings of the XLIX Italian Society of Agricultural
Genetics, Annual Congress, Potenza, Italy. 2005: 28
16. Harman
GE, Howel, CR, Viterbo A,
Chet I, and Lorito M. Trichoderma
species- Opportunistic, avirulent plant symbionts. Nature Rev Microbiol,
2004; 2: 43-56.
17. Zimand
G, Elad Y, and Chet I. Effect of Trichoderma harzianum on Botrytis cinerea pathogenicity.
Phytopathol, 1996; 86: 1255-1260.
18. Elad
Y. Biological control of foliar pathogens by means of Trichoderma
harzianum and potential modes of action. Crop Prot, 2000; 19: 709-714.
19. Pandey
A. Antagonism of two Trichoderma
species against Alternaria alternata on Capsicum frutescens.
J Exp Sci,
2010; 1 (5): 18-19.
20. Thaware DS, Fugro PA, Jadhav
YT, Magar SV, and. Karande
RA.. In vitro evaluation of different fungicides, plant extracts and
bio-agents against Alternaria alternata (Fr.) Keissler
causing leaf blight of cowpea. Int J Plant Prot, 2010; 3(2): 356-360.
21. Ambuse
MG, Chatage VS, and Bhale
UN. Influence of trichoderma
spp against alternaria
tenuissima inciting leaf spot of rumex acetosa. Biosci Discov. 2012; 3(2): 259 -262.