Int. J. Life. Sci.
Scienti. Res., 4(6): 2111-2115, November 2018
Growth
Curve Analysis of Rhizobium leguminosarum Using Voltage Produced by
Microbial Fuel Cell
Shrirang R. Maddalwar1*, Dr. Arti S. Shanware2
1Student, Department of Biotechnology, Rajiv Gandhi Biotechnology
Centre, Nagpur, India
2Director, Department of Biotechnology, Rajiv Gandhi Biotechnology
Centre, Nagpur, India
*Address for Correspondence: Mr. Shrirang R. Maddalwar, Student,
Department of Biotechnology, Rajiv Gandhi Biotechnology Centre, Nagpur-440020,
India
ABSTRACT - Microbial fuel cells could be used to
the study growth rates of aerobic microbial species on the basis of voltage
produced by them in the microbial fuel cell assembly. A fresh culture of Rhizobium
leguminosarum was added in the anode chamber of a microbial fuel cell
assembly and subsequent voltage produced by it was recorded after every fifteen
minutes. The 24 ml/hr of air was pumped in the anode chamber to maintain the dissolved
oxygen level and resistance of 12 ohm was applied across the electrodes. This
process was studied in triplicates and voltage data was recorded. The graph
plotted of voltage against time suggested the growth curve of the species in
the microbial fuel cell system. It was found that voltage gradually increased
with time ranging from 50 mV to 190 mV with a supply of oxygen in the anode,
but it declines gradually to zero in absence of aeration with time and
depletion of nutrients.
Keywords- Rhizobium leguminosarum,
Exo-electrogenesis, Microbial fuel cell, Sporulation, Proton
exchange membrane
INTRODUCTION- It is really difficult task to study metabolic activities of a
single bacterial cell, hence in order to avoid this problem the culture is
usually manipulated in such a way that all the cells of culture should be
showing same metabolic activities. This method is very useful in physiological
studies and also known as synchronous culture method [1-3]. A study
of these cells would enable one to postulate the sequence of events occurring
in a single cell during the process of sporulation [4]. Though this
method is very effective to study the metabolic activities of Rhizobium leguminosarum but
it fails to convey the required information when the culture is not synchronous
[5].
A Microbial Fuel Cell (MFC)
is a device that converts chemical energy from bio-convertible organic
substrate, directly into electrical energy through the metabolic activity of
microorganisms. A simple MFC setup contains two chambers respectively anode and
cathode separated by Proton Exchange Membrane (PEM). The microorganisms are
inoculated in an anodic chamber, where they oxidize the substrate and generate
protons and electrons. The electrons are transferred from anode to cathode
through the external circuit and the protons pass through the proton exchange
membrane to cathode, where the proton meets the oxygen and electrons to form
water [6].
Mechanism of electron
transformation from bacterial cell to the anode is known by three ways,
firstly, using exogenous mediators (those present outside the cell) such as
thionine, methylene blue or neutral red and potassium ferricyanide. Secondly,
using mediators produced by the bacteria and finally by direct transfer of
electrons from the respiratory chain enzymes i.e. cytochromes, to the outer
cell membrane, which in turn is reduced and then leaving it in a reduced state
to shuttle the electrons to the electrode [7]. Geobacter
sulfurreducens, Geobacter metallireducens, and Rhodoferax
ferrireducens have been shown to produce the voltage in a mediator
less microbial fuel cell [8].
Hence, in order to study
metabolic activities of Rhizobium
leguminosarum in liquid broth, the microbial fuel cell could
be used as an effective tool. This study focuses on voltage generated by the
microbial fuel cell using fresh broth of Rhizobium leguminosarum with respect
to aeration and in absence of aeration. It could be an effective method to
determine the metabolic rate of cells in different physiological and
nutritional conditions.
MATERIAL AND METHODS
Pre-isolated and
pre-characterized culture tube of Rhizobium
leguminosarum from the soil samples Rajiv Gandhi
Biotechnology Centre, Nagpur, India premises was considered for inoculation of
fresh growth medium in laboratory of Department of Biotechnology at 16 December
2017.
Preparation of fresh culture
broth- The fresh nutrient medium was prepared
using Yeast Mannitol medium (YEMA Medium) which includes K2HPO4 0.05%, MgSO4
0.02%, NaCl 0.01%, Mannitol 1%, CaCO3 0.3%, Yeast 0.1% and Distilled
water. The contents of the medium are thoroughly mixed and allowed for
autoclaving at 121oC and 15 lb pressure. After the process of autoclaving
the medium, the medium is allowed to settle down to room temperature. This
process can be done more rapidly by placing the container of nutrient medium in
cool stream of water. Once the content is cooled then pH of the medium was
measured using digital pH meter. The optimum pH of this medium for Rhizobium
species should be 7 and hence it is necessary to confirm it. If the pH is not
neutral, then it is made neutral by adding strong acid like HCl or strong base
like NaOH. After gaining optimum pH, the broth was inoculated with 10%
inoculums of a Rhizobium leguminosarum and the content was
allowed to grow in well-aerated desktop fermentor or cotton-plugged glass
flask. Culture requires oxygen to multiply and grow efficiently; hence aerobic
fermentor is always preferable. The medium was allowed to grow for next 48
hours [9].
Microbial fuel cell construction- The MFC was constructed using two
screw-capped plastic bottles with the total working volume of 1 liter and it
served as anode (anaerobic) and cathode (aerobic) chambers. Both anode and
cathode chambers were connected with 1 cm in diameter and 5 cm long tube which
was filled up with salt bridge made of 1M Potassium Chloride (KCl) solution and
3% agar powder. Agar salt bridge acts as a barrier between the anode and
cathode chambers. The reason for using agars as salt bridge is to provide an
internal electrical connection between the chambers, while minimizing the
transfer of ions from the electrical environment [10]. Stainless
steel mesh of 500 gm and having diameter of 2 mm was used as anode and cathode.
Before the MFC operation, the electrodes were soaked in 0.1 M HCl solution for
a day to remove possible contamination and after the MFC operation the electrodes were washed with
0.1 M NaOH solution to neutralize the surface contaminants [11]. The electrodes were externally connected with 12 ohm resistance
using copper wire. This setup was prepared in triplicates in order to minimize
the errors in voltage recorded by the volt meter. Aeration of 600 ml per hour
is supplied to the cathode chamber using 1 liter injection syringe. Both the
bottles are screw capped properly in order to make both the chambers air tight.
Microbial fuel cell
operation- All the three microbial fuel cell setups are surface sterilized by
wiping with 70% alcohol and UV light exposure for 20 minutes. All the three
anode chambers of microbial fuel cell assemblies were filled with 400 ml
freshly prepared broth culture of Rhizobium leguminosarum in each chamber. After inoculation, the
assemblies are screw capped properly to maintain anaerobic conditions inside
the chamber. In cathode chamber, 800 ml of 1 M KCl solution was filled in all
the three assemblies. All the three assemblies are screw capped and aeration of
600 ml per hour is provided to the assemblies using 1 liter injection syringe.
Whatman filter is attached to the syringe before aeration in order to provide
sterile air flow [9].
RESULTS- A resistance of 12 ohm
is applied across cathodes and anodes of each assembly and voltage is recorded
in the voltmeter after every fifteen minutes. After one hour and 45 minutes,
the aeration is stopped and voltage is recorded after every 15 minutes for five
more times successively. Detailed data of recorded voltage in mV is summarized
in Table 1 and Fig. 1.
Table 1: Voltage
recorded by three microbial fuel cell assemblies after every 15 minutes
|
Time (Minutes) |
Voltage recorded by Set A (mV) |
Voltage recorded by Set B (mV) |
Voltage recorded by Set C (mV) |
|
0 |
90 |
50 |
70 |
|
15 |
130 |
70 |
100 |
|
30 |
130 |
90 |
120 |
|
45 |
160 |
108 |
127 |
|
60 |
185 |
130 |
135 |
|
75 |
184 |
145 |
150 |
|
90 |
184 |
180 |
182 |
|
105 |
184 |
180 |
182 |
|
AERATION |
STOPPED |
||
|
120 |
190 |
190 |
180 |
|
135 |
180 |
190 |
170 |
|
150 |
150 |
180 |
120 |
|
165 |
140 |
144 |
108 |
|
180 |
130 |
25 |
80 |
|
195 |
10 |
00 |
00 |
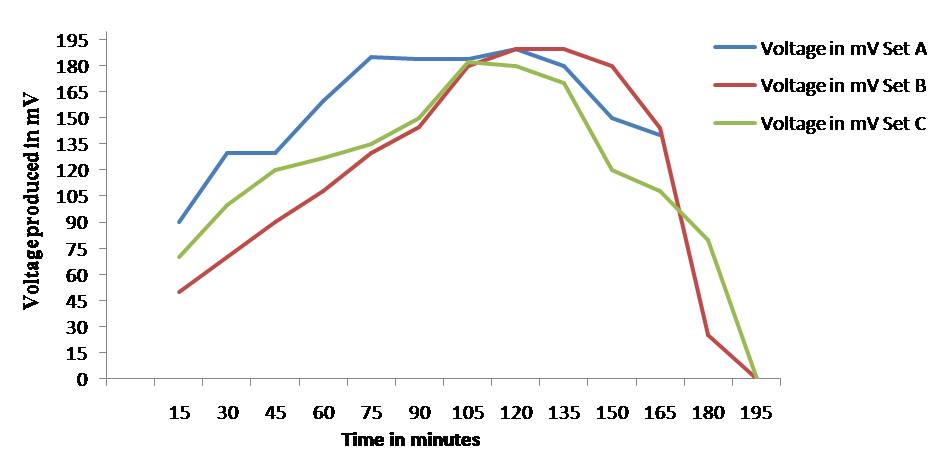
Fig. 1: Voltage produced by the culture of Rhizobium species with
respect to time
DISCUSSION- There has been an increase
in recent years in the number of reports of microorganisms that can generate
electrical current in microbial fuel cells. Although many new strains have been
identified, few strains individually produce power densities as high as strains
from mixed communities. Enriched anodic bio-films have generated power
densities as high as 6.9 W per m2 (projected anode area), and therefore are
approaching theoretical limits [12].
Power density, electrode
potential, coulombic efficiency, and energy recovery in single-chamber
microbial fuel cells (MFCs) were examined as a function of solution ionic
strength, electrode spacing and composition, and temperature. By the increasing
the solution ionic strength from 100 to 400 mM by adding NaCl increased power
output from 720 to 1330 mW/m2. Power generation was also increased
from 720 to 1210 mW/m2 by decreasing the distance between the anode and cathode
from 4 to 2 cm. The power increases due to ionic strength and electrode spacing
resulted from a decrease in the internal resistance. Power output was also
increased by 68% by replacing the cathode (purchased from a manufacturer) with
carbon cloth cathode containing the Pt loading [13].
It is a surprise to many
researchers that the most significant block to achieving high power densities
in MFCs is the system architecture, not the composition of the bacterial
community [14].
But power output by MFCs
has been consistently increasing over time. Improvements in system architecture
and operation have increased power densities from 1500 mW/m2 using
oxygen as the final electron acceptor at the cathode [15,16].
From Fig. 1 and Table 1 were observed that
the voltage produced by a freshly prepared broth of Rhizobium leguminosarum increases with time from its actual value to
180 - 190 mV with aeration of 600 ml per hour of sterile air in cathode chamber
in 105 minutes. But when the aeration is stopped completely, the voltage
produced by the culture in microbial fuel cell setup also decreases gradually
and it becomes zero after 180 minutes of starting the experiment. This change
in voltage with respect to time and with respect to aeration observed in very
similar pattern in all the three sets namely A, B, and C or microbial fuel cell
assembly. Hence, the graph of voltage against time gradually increases with
aeration and it gradually decreases from the peak when the aeration is stopped
in the cathode chamber of microbial fuel cell assemblies. The graph declines to
zero after 180 minutes.
The increase in the voltage generated by
microbial fuel cells with aeration suggests that metabolic rate of the growing
culture in the anode chamber increased with aeration, but after stoppage of
aeration, the metabolic rate of the bacterial culture decreased gradually due
to insufficiency of oxygen in the cathode chamber and as a result voltage
produced by it also decreased and it become zero after 195 minutes of
experiment when oxygen was completely exhausted in the cathode chamber. Zero
voltage recorded by microbial fuel cell assemblies suggests that the fuel cell
stops working and cells in the culture might have died.
CONCLUSIONS- From this study it could be concluded that
pre-isolated strain of Rhizobium leguminosarum is highly aerobic in nature and require enough oxygen to metabolize and reproduce.
The cell behavior or nutrient uptake studies of Rhizobium leguminosarum could be done using the voltage generated by
it in microbial fuel cell assemblies. The maximum voltage generated by a
freshly prepared broth of Rhizobium leguminosarum is in between 180 mV - 190 mV in microbial
fuel cell assembly.
In future, more work is required to be done
on the assembly for increasing this range of voltage. In addition to this
comparative study of different electrode material can also be done in order to
get an efficient and steady voltage for the sample organism.
ACKNOWLEDGEMENT- This investigation was fulfilled with the continuous support of
all the teaching and non-teaching staff of Rajiv Gandhi Biotechnology Centre,
Nagpur. I am especially thankful to Dr. Arti S. Shanware for her continuous
guidance and support while undergoing this research work.
CONTRIBUTION OF AUTHORS - All authors
equally contributed to this article.
REFERENCES
1.
Halvorson HO. Physiology of
sporulation, In I. C. Gunsalus and R. Y. Stanier [ed.], the bacteria. Academic
Press, Inc., New York, 1962; 4: 223-274.
2.
Halvorson HO. Sequential
expression of biochemical events during intracellular differentiation. Symp.
Soc. Gen. Microbiol., 1965; 15: 343-368.
3.
Murrell WG. Spore formation and
germination as a microbial reaction to the environment. Symp. Soc. Gen.
Microbiol., 1961; 11: 100-150.
4.
Microbial fuel cells. USA.
John Wiley and Sons, 2008; pp. 1-125.
5.
Imanaka H, Gillis JR,
Slepecky RA. Synchronous Growth and Sporulation of Bacillus megaterium. J Bacteriol., 1967; 93(5): 1624-1630.
6.
Sharma Suresh K, Bulchandani
BD. Comparative Study of Various Substrates and Microorganisms in a Laboratory
Designed Microbial Fuel Cell Int. J Res Chem Environ. 2012; 2(3): 168-174.
7.
Chaudhury SK, Lovely DR.
Electricity generation by direct oxidation of glucose in mediatorless microbial
fuel cells. Nat. Biotechnol., 2003; 21: 1229-1232.
8.
Bond DR, Lovely DR.
Electricity production by Geobacter sulfurreducens attached to electrodes,
Appl Environ Microbiol., 2003; 69: 1548-1555.
9.
Jakaria
Al-Mujahidy Sk, Hassan M, Rahman M and Mamun-Or-Rashid ANM Isolation and
characterization of Rhizobium sp. and determination of their potency for growth
factor production. Int. Res. J. Biotechnol., 2013; 4(7): 117-123.
10.
Patil
VD, Patil DB, Deshmukh MB, Pawar SH. Comparative study of bioelectricity
generation along with the treatment of different sources of wastewater.
International Journal of Chemical Sciences and Applications, 2011; 2(2): 162-168.
11.
Liu ZD,
Li HR. Effects of Bio and Abio-factors on Electricity Production in a
Mediatorless Microbial Fuel Cell, Biochem Eng J., 2007; 36(3): 209-214.
12.
Logan
BE. Exoelectrogenic bacteria that power microbial fuel cell. Nature reviews:
microbiology, 2009, pp. 375-381.
13.
Hong
Liu, Shaoan Cheng, Logan BE. Power generation in Fed- Batch Microbial Fuel Cell
as a function of ionic strength, Temperature, & Reactor Configuration. Environ.
Sci. Technol. 2005; 39 5488-5493.
14.
Kim BH, et al. Mediator-less biofuel cell.
U.S. Patent 5976719, 1999.
15.
Cheng
S, et al. Increased power generation in a continuous flow MFC with advective
flow through the porous anode and reduced electrode spacing. Environ. Sci.
Technol., 2006; 40: 2426–2432,
16.
Min B,
et al. Electricity generation using membrane and salt bridge microbial fuel
cells. Water Res., 2005; 39, 1675–1686.