Int. J. Life. Sci. Scienti. Res., 4(5):
2044-2055,
September 2018
Analysis
of Changing Vegetation Pattern Under Different Climatic, Edaphic and
Altitudinal Factors of Doon Valley, Uttarakhand, India
Narendra Kumar1*, Kartik
Uniyal2, Zakir Nazir3
1Assistant
Professor cum Head, Department of Botany, Alpine Institute of Management &
Technology, Dehradun, Uttarakhand, India
2Assistant
Professor cum Head, Department of Biotechnology & Microbiology, Alpine
Institute of Management & Technology, Dehradun, Uttarakhand, India
3Student,
Department of Botany, Alpine Institute of Management & Technology,
Dehradun, Uttarakhand, India
*Address for
Correspondence: Dr. Narendra
Kumar, Assistant Professor cum Head, Department of Botany, Alpine Institute of
Management & Technology, Dehradun, Uttarakhand -248 007, India
ABSTRACT
- The present study was conducted in altitudinal variable hight and soil pH
growing vegetation of Doon valley that the soil type found in Mussoorie is generally
medium loamy but its composition, moisture, and pH generally varied from place
to place. Higher silt in Mussoorie was due to higher precipitation in the form
of winter snow above 1800 m. Soil temperature in Mussoorie ranged from 40C-160C,
while that of Sahastradhara and sudhowala was 15-190C. Soil organic
matter content tended to be higher in high altitude and increased with
increasing altitude. In this study, our finding observed that Soil temperature
in Mussoorie ranged from 40C-160C, while that of
Sahastradhara was 15-190C, Sudhowala 15-250C. Soil
organic matter content tended to be higher in increase high altitudinal
gradient. As per altitudinal variation
and soil pH in this region dominant family of angiosperms in Garhwal Himalaya
are Asteraceae, Brassicaceae, Cyperaceae, Fabaceae, Lamiaceae, Poaceae,
Ranunculaceae, Polygonaceae, Amaranthaceae, Solanaceae, Sexifragaceae,
Ranunculaceae Orchidaceae, Apocynaceae, Rutaceae, and Rosaceae.
Keywords: Altitude,
Altitudinal Factors Angiosperms, Phytodiversity, Species analysis, Vegetation
Pattern, Edaphic
INTRODUCTION-
Himalaya
being the richest biogeographically zones in India is provided with diverse
vegetation. The location, climate, topography and other environmental factors
of Himalaya have enriched it with diverse life forms. The Himalaya blooming
with rivers, frozen glaciers, high mountain peaks that remain loaded with snow
for most of the time, evergreen lakes, with the enormous diversity of flora
(50% of the Indian subcontinent) is rightly called as the “Abode of God”. Due
to cyclic climate changes mainly by anthropogenic activities, floral diversity
of Himalaya is influenced to a large extent.
Garhwal
Himalaya is one among the most fascinating segment of a Himalayan arc and
unique in its geology. Garhwal Himalaya is a great attraction to geologists and
ecologists from all over the world because of its richness in biodiversity and
it is the confluence of all rock formations resulting in different soil types
and hence diverse vegetation types [1].
The
Climate of Garhwal Himalaya varies from dry to moist conditions which have a
great influence on growth, vitality, and distribution of floristic vegetation.
Garhwal Himalaya is a mass of intricate folding and faulting and is composed of
igneous, sedimentary and metamorphic rocks [2]. Garhwal Himalaya has been extensively surveyed in
terms of vegetation by many workers and floral diversity has been explored to a
large extent [3,4], since the native floristic biodiversity of these
restored sites of Doon valley is facing serious threats from anthropogenic
activities, urbanization, and climate change, so the main aim of the study was
to analyze the present status of the angiospermic vegetation of these sites.
MATERIALS AND METHODS
Study site-
The present study was conducted in degraded and restored limestone mines of
Doon valley, viz: Sudhowala, Sahastradhara, and Mussoorie. The areas were
observed phytosociologically during February to May 2017.
Doon
Valley (Area 3008 sq. km) is situated between foothills of Garhwal Himalaya and
Shiwaliks at an altitude of 2200 ft. above sea level. The area lies between
30.3840 N and 77.9739 E and receives an annual rainfall of 2073.3 mm. The east
and west boundaries of Doon valley are limited by rivers Ganga and Yamuna
respectively.
The
Climate of an area is temperate although it varies from tropical to cold
depending on the season and altitude. The average maximum and minimum
temperatures are 27.650C and 13.80C respectively [5].
The study area was divided into three sub-areas viz. Sudhowala, Sahastradhara,
and Mussoorie. In the study site, different types of angiospermic families were
recorded which show biodiversity variability with varying Soil pH and climatic
condition.
Sampling- Soil samples were collected from three
different sites (Sudhowala, Sahastradhara, and Mussoorie) from uppermost part
(5”-8”) as well as dried properly and preserved for pH measurement.
Soil temperature measurement- The
study area was repeatedly visited to measure the soil temperature by a soil
thermometer. Temperature measurement was done on 26th of Feb and 5th
of March in Mussoorie, 3rd of March and 15th of March in
Sahastradhara and 20th of Feb, 08th of March and 18th
of March in Sudhowala.
Field survey and Data Collection- Extensive
field surveys were conducted repeatedly from February to April in all the three
different sites viz Sudhowala, Sahastradhara, and Mussoorie to observe the
natural habitats and to collect the plant specimens. Various other parameters
such as altitude, measurement of soil temperature and vegetation types were
recorded. For plant identification purposes herbarium of Forest Research
Institute of India and herbarium of Botanical Survey of India northern region
were used. Plant specimens were classified on the basis of habit and life forms
as by Raunkiaer [6] and distribution of pattern follows as per Odum
[7].
Soil sample collection and
Measurement of soil pH- Soil samples collected from all the
three different sites were dried properly and sieved (2 mm). Different soil
solutions were made by dissolving soil in distilled water. Analysis of soil pH
and altitudinal variation was done as described by Schoenholtza et al. [8]; Raina and Gupta
[9]; Arya [10].
RESULTS-
Garhwal
Himalayan is one of the most fascinating segments of the Himalayan arc and is
unique for its geological setting, and so the unique floristic vegetation. A
variety of factors contribute to the diversity of floristic vegetation in the
study area. The dominance of Lantana
camara, Ageratum conyzoides, Eupatorium adenophorum, Parthenium hysterophorus,
Mallotous phillipensis, Shorea robusta, Amaranthus spinosus, Euphorbia hirta,
Rumex hastatus, and Clerodendrum
viscosum in the study area possibly shows the availability of optimum
conditions for their growth. The uniform abundance of Lantana camera, Ageratum conyzoides, and Parthenium hystoriphorus is due to their environmental plasticity,
as they are shade and light tolerant.
Soil pH of different regions of
Doon Valley- Soil samples were collected from
Mussoorie, Sahastradhara, and Sudhowala regions of Doon valley during February
to April 2017. Soil samples were collected from the top soil (15 cm depth).
Soil samples were stored under proper conditions and then were used for pH test
by using Glass electrode pH meter. Soil temperature was measured by a soil
thermometer.
(a) In Sudhowala region of the study area,
soil pH varied from 5.4-7.40.
(b) In Sahastradhara region of study area,
soil pH ranged from 5.5-7.80.
(c)
In Mussoorie region of the study area, soil pH ranged from 5.2-8.20.
The
possible reason for the low pH value of Sudhowala soil sample is due excessive
leaching of minerals as compared to adjacent areas of Doon valley. The pH
differed significantly between land use systems; soil pH of natural woodlands
is lower than of remaining land use systems. During the study period the
angiospermic plant diversity which includes herbs, shrubs, climbers, and trees
at changing pH patterns in three different communities of Doon valley, the
dicot families were found in abundance as compared to monocot families which
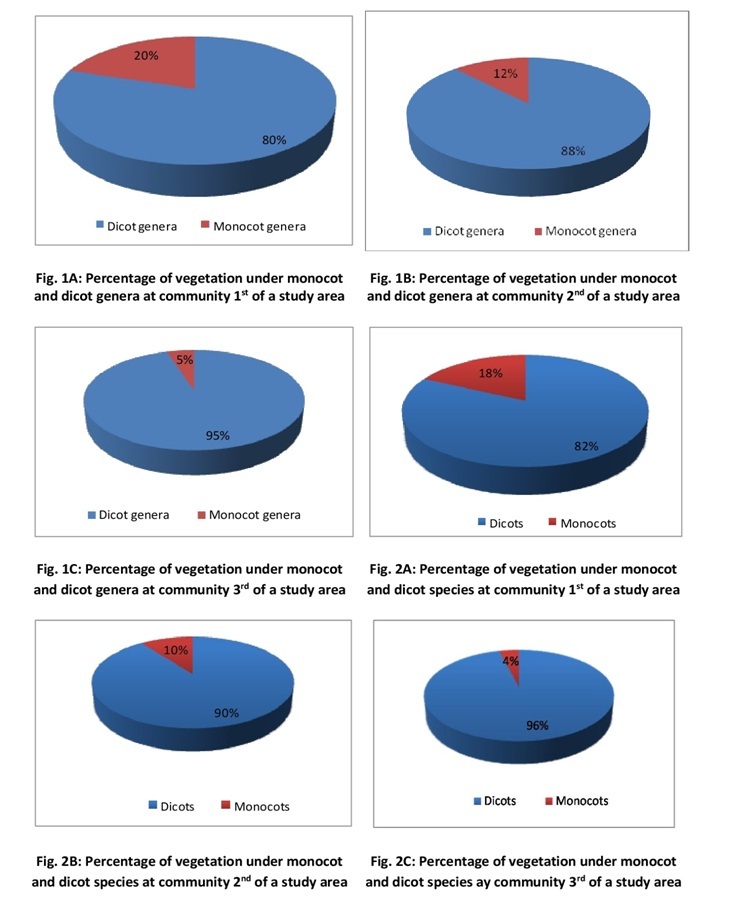
contribute a little percentage.
In
community 1st as many as 33 angiospermic families having 62 genera and
76 species were observed, of which 7 were monocot families viz. Poaceae,
Musaceae, Orchidaceae, Cannaceae, Zingiberaceae, and Liliaceae. Among monocot
families, Poaceae is represented by the highest number of species (6). The
important members of the family found in the study area are Cyanodon dactylon, Poa annua, Triticum
aestivum, Dendrocalmus giganteus, and
Saccharum officinarium. Dicots
were mainly represented by Malvaceae, Solanaceae, Euphorbiaceae, Asteraceae,
Convolvulaceae, and Verbinaceae. Among dicots, Solanaceae showed the highest
number of species (6) and is mainly represented by Datura stramonium, Solanum torvum, and Solanum melongena.
In
community 2nd a total of 35 angiospermic families having 59 genera
and 65 species were observed, of which monocots were represented by only two
families viz. Poaceae, and Cyperaceae. Poaceae showed the highest number of
species, the important among them are Apluda
mutica, Cynodon dactylon, Poa annua and Saccharum officinarium.
Cyperaceae is represented by a single species Cyperus rotundus. Among Dicot families Solanaceae and Asteraceae
showed the highest number of species (5 each). The important members of
Solanaceae found in the area are Solanum
nigrum, Lycopersicon esculentum, and Datura stramonium. The important species of the Asteraceae family
found in the area are Eupatorium
adenophorum, Xanthium indicium, Ageratum conyzoides, and Bidens biternata.
Furthermore,
community 3rd also showed similar results i.e. dominance of dicot
families. A total of 30 families having 63 genera and 75 species of angiosperms
and were observed, of which monocots were represented by only 3 families viz.
Cyperaceae, Poaceae, and Melanthiaceae, all represented by a single species viz. Cyperus rotundus, Cyanodon dactylon, and Paris
polyphylla respectively. Among dicots, family Rosaceae is represented by
the highest number of species (12), the important members of the family found
in the study area are Fragaria indicia,
Rubus ellipticus, Rosa moschata, Prunus persica, and Pyrus pashia. Some other important dicot
families found in this community are Ranunculaceae, Fabaceae, Lamiaceae,
Malvaceae, Salicaceae, Rubiaceae, Asteraceae, Solanaceae, and Polygonaceae. At
changing altitude and soil pH of three different sites of Doon valley, the
angiospermic vegetation of different sites is given in three separate lists.
Table 1: Community first shown the
following angiospermic plant species at a pH range of 5.4-7.40
|
S.
No |
Botanical name |
Family |
Division |
|
1.
|
Mangifera indica |
Anacardiaceae |
Dicot |
|
2.
|
Anacardium accidentale |
Anacardiaceae |
Dicot |
|
3.
|
Gossypium hirsutum |
Malvaceae |
Dicot |
|
4.
|
Sida acuta |
Malvaceae |
Dicot |
|
5.
|
Sida cordata |
Malvaceae |
Dicot |
|
6.
|
Datura stramonium |
Solanaceae |
Dicot |
|
7.
|
Solanum torvum |
Solanaceae |
Dicot |
|
8.
|
Lycopersicum esculentum |
Solanaceae |
Dicot |
|
9.
|
Capsicum sp. |
Solanaceae |
Dicot |
|
10. |
Solanum xanthocarpus |
Solanaceae |
Dicot |
|
11. |
Solanum melongena |
Solanaceae |
Dicot |
|
12. |
Brassica oleracea |
Brassicaceae |
Dicot |
|
13. |
Brassica napobrassica |
Brassicaceae |
Dicot |
|
14. |
Raphanus sativus |
Brassicaceae |
Dicot |
|
15. |
Brassica rapa |
Brassicaceae |
Dicot |
|
16. |
Prunus persica |
Rosaceae |
Dicot |
|
17. |
Prunus domestica |
Rosaceae |
Dicot |
|
18. |
Mallotous philippinensis |
Euphorbaceae |
Dicot |
|
19. |
Euphorbia hirta |
Euphorbaceae |
Dicot |
|
20. |
Ricinus communis |
Euphorbaceae |
Dicot |
|
21. |
Musa accuninata |
Musaceae |
Monocot |
|
22. |
Phaseolus vulgaris |
Fabaceae |
Dicot |
|
23. |
Pisum saitivum |
Fabaceae |
Dicot |
|
24. |
Glycine max |
Fabaceae |
Dicot |
|
25. |
Desmodium gangeticum |
Fabaceae |
Dicot |
|
26. |
Trifolium repens |
Fabaceae |
Dicot |
|
27. |
Citrus aurantium |
Rutaceae |
Dicot |
|
28. |
Citrus limonum |
Rutaceae |
Dicot |
|
29. |
Murraya koenigii |
Rutaceae |
Dicot |
|
30. |
Carica papaya |
Caricaceae |
Dicot |
|
31. |
Lantana camara |
Verbinaceae |
Dicot |
|
32. |
Clerodendrum viscosum |
Verbinaceae |
Dicot |
|
33. |
Parthenium hysterophorus |
Asteraceae |
Dicot |
|
34. |
Ageratum conyzoides |
Asteraceae |
Dicot |
|
35. |
Xanthium indicum |
Asteraceae |
Dicot |
|
36. |
Eupatorium adenophorum |
Asteraceae |
Dicot |
|
37. |
Artemisia parviflora |
Asteraceae |
Dicot |
|
38. |
Callistemon lanceolatus |
Myrtaceae |
Dicot |
|
39. |
Morus alba |
Moraceae |
Dicot |
|
40. |
Bougainvillea sp. |
Nyctaginaceae |
Dicot |
|
41. |
Saccharum officinarium |
Poaceae |
Monocot |
|
42. |
Dendrocalamus giganteus |
Poaceae |
Monocot |
|
43. |
Polypogon fugax |
Poaceae |
Monocot |
|
44. |
Triticum spp. |
Poaceae |
Monocot |
|
45. |
Poaannua |
Poaceae |
Monocot |
|
46. |
Cyandon dactylon |
Poaceae |
Monocot |
|
47. |
Agave sisalana |
Asparagaceae |
Monocot |
|
48. |
Aspergus recemosus |
Asparagaceae |
Monocot |
|
49. |
Ocimum sanctum |
Limaceae |
Dicot |
|
50. |
Mantha longifolia |
Limaceae |
Dicot |
|
51. |
Rheum rhaponticum |
Polygonaceae |
Dicot |
|
52. |
Polygonium barbatum |
Polygonaceae |
Dicot |
|
53. |
Polygonium hydropiper |
Polygonaceae |
Dicot |
|
54. |
Daucus carota |
Apiaceae |
Dicot |
|
55. |
Apium graveolens |
Apiaceae |
Dicot |
|
56. |
Piper nigrum |
Piperaceae |
Dicot |
|
57. |
Vanilla planiflora |
Orchidaceae |
Monocot |
|
58. |
Calotropis procera |
Apocynaceae |
Dicot |
|
59. |
Thevetia paruviana |
Apocynaceae |
Dicot |
|
60. |
Catharanthus roseus |
Apocynaceae |
Dicot |
|
61. |
Ipomoea batates |
Convolvolaceae |
Dicot |
|
62. |
Ipomoea aquatic |
Convolvolaceae |
Dicot |
|
63. |
Ipomoea nil |
Convolvolaceae |
Dicot |
|
64. |
Canna indica |
Cannaceae |
Monocot |
|
65. |
Chenopodium album |
Amaranthaceae |
Dicot |
|
66. |
Amaranthus spinosus |
Amaranthaceae |
Dicot |
|
67. |
Pyrostegia venusta |
Binoniaceae |
Dicot |
|
68. |
Tecoma castanifolia |
Bignoniaceae |
Dicot |
|
69. |
Delphenium denudatum |
Rannunculaceae |
Dicot |
|
70. |
Shorea robusta |
Dipterocarpaceae |
Dicot |
|
71. |
Cannabis sativa |
Canabaceae |
Dicot |
|
72. |
Allium cepa |
Alliaceae |
Monocot |
|
73. |
Circuma longa |
Zingiberaceae |
Monocot |
|
74. |
Calotropis procera |
Asclpedaceae |
Dicot |
|
75. |
Papaver somniferum |
Papaveraceae |
Dicot |
|
76. |
Argemone mexicana |
Papaveraceae |
Dicot |
Table
2: Community 2nd shown the following angiospermic plant species at pH
range of 5.5-7.80
|
S.
No |
Botanical name |
Family |
Division |
|
1 |
Eupatorium adenophorum |
Asteraceae |
Dicot |
|
2 |
Bidens
biternata |
Asteraceae |
Dicot |
|
3 |
Parthenium hystrophorus |
Asteraceae |
Dicot |
|
4 |
Xanthum indicum |
Asteracaea |
Dicot |
|
5 |
Ageratum conzoides |
Asteraceae |
Dicot |
|
6 |
Lantana camara |
Verbinaceae |
Dicot |
|
7 |
Clerodendrum viscosum |
Verbinaceae |
Dicot |
|
8 |
Solanum nigrum |
Solanaceae |
Dicot |
|
9 |
Solanum torvum |
Solanaceae |
Dicot |
|
10 |
Solanum melongena |
Solanaceae |
Dicot |
|
11 |
Datura stramonium |
Solanaceae |
Dicot |
|
12 |
Lycopersicon esculentum |
Solanaceae |
Dicot |
|
13 |
Achyranthus aspera |
Amaranthaceae |
Dicot |
|
14 |
Aerva sanguinolenta |
Amaranthaceae |
Dicot |
|
15 |
Amaranthus spinosus |
Amaranthaceae |
Dicot |
|
16 |
Chenopodium album |
Chenopodiaceae |
Dicot |
|
17 |
Cynodon dactylon |
Poaceae |
Monocot |
|
18 |
Poa annua |
Poaceae |
Monocot |
|
19 |
Triticum aestivum |
Poaceae |
Monocot |
|
21 |
Apluda mutica |
Poaceae |
Monocot |
|
22 |
Pennisetum flaccidum |
Poaceae |
Monocot |
|
23 |
Saccharum officinarium |
Poaceae |
Monocot |
|
24 |
Cyperus rotundus |
Cyperaceae |
Monocot |
|
25 |
Bombax cieba |
Bombacaceae |
Dicot |
|
26 |
Utrica dioca |
Utricaceae |
Dicot |
|
27 |
Boehmeria plahyphylla |
Utricaceae |
Dicot |
|
28 |
Brassica compestris |
Brassicaceae |
Dicot |
|
29 |
Polygonium hydropiper |
Polygonaceae |
Dicot |
|
30 |
Polygonium barbatum |
Polygonaceae |
Dicot |
|
31 |
Rumex hastatus |
Polygonaceae |
Dicot |
|
32 |
Lepidagathis incurva |
Acanthaceae |
Dicot |
|
33 |
Adhatoda vasica |
Acanthaceae |
Dicot |
|
34 |
Sida cordata |
Malvaceae |
Dicot |
|
35 |
Sida acuta |
Malvaceae |
Dicot |
|
36 |
Sida rhombiflora |
Malvaceae |
Dicot |
|
37 |
Sida cordifolia |
Malvaceae |
Dicot |
|
39 |
Nepeta hindostania |
Lamiaceae |
Dicot |
|
40 |
Ajuga bracteosa |
Lamiaceae |
Dicotf |
|
41 |
Ocimum sanctum |
Lamiaceae |
Dicot |
|
42 |
Ipomoea nil |
Convolvulaceae |
Dicot |
|
43 |
Booerhaavia diffusa |
Nyctaginaceae |
Dicot |
|
44 |
Rubus ellipticus |
Rosaceae |
Dicot |
|
45 |
Fragaria indica |
Rosaceae |
Dicot |
|
46 |
Dalbaragia sisoo |
Fabaceae |
Dicot |
|
47 |
Desmodium gangeticum |
Fabaceae |
Dicot |
|
48 |
Flemingia stroblifera |
Fabaceae |
Dicot |
|
49 |
Reinwardtia indica |
Linaceae |
Dicot |
|
50 |
Anagallis arvensis |
Primulaceae |
Dicot |
|
51 |
Stellaria media |
Caryophyllaceae |
Dicot |
|
52 |
Oxalis corniculata |
Oxalidaceae |
Dicot |
|
53 |
Pellucida pepromia |
Piperaceae |
Dicot |
|
54 |
Mallotus philippensis |
Euphorbaceae |
Dicot |
|
55 |
Ricinus communis |
Euphorbaceae |
Dicot |
|
56 |
Jatropha curcas |
Euphorbaceae |
Dicot |
|
57 |
Aspergus filicinus |
Aspergaceae |
Dicot |
|
58 |
Murraya koenigii |
Rutaceae |
Dicot |
|
59 |
Acacia catechu |
Mimosaceae |
Dicot |
|
60 |
Carissia opaca |
Apocynaceae |
Dicot |
|
61 |
Centella asiatica |
Apiaceae |
Dicot |
|
61 |
Salix tetrasperma |
Salicaceae |
Dicot |
|
62 |
Randia spinosa |
Rubiaceae |
Dicot |
|
63 |
Woodfordia fruticosa |
Lythraceae |
Dicot |
|
64 |
Vitis himalayana |
Vitaceae |
Dicot |
|
65 |
Ranunculus scleratus |
Rannunculaceae |
Dicot |
Table
3: Community 3rd shown following angiospermic plant species at a pH
range of 5.2-8.20
|
S.
No |
Botanical name |
Family |
Division |
|
01 |
Delphanium denudatum |
Rannunculaceae |
Dicot |
|
02 |
Clematis Montana |
Rannunculaceae |
Dicot |
|
03 |
Rannunculus laetus |
Rannunculaceae |
Dicot |
|
04 |
Rannunculus hirtellus |
Rannunculaceae |
Dicot |
|
05 |
Berberis asiatica |
Rannunculaceae |
Dicot |
|
06 |
Schisandra grandiflora |
Schisandraceae |
Dicot |
|
07 |
Argemone mexicana |
Papaveraceae |
Dicot |
|
08 |
Stellaria media |
Caryophyllacea |
Dicot |
|
09 |
Malva neglecta |
Malvaceae |
Dicot |
|
10 |
Trifolium repens |
Fabaceae |
Dicot |
|
11 |
Astragalus trichocarpus |
Fabaceae |
Dicot |
|
12 |
Desmodium multiflorus |
Fabaceae |
Dicot |
|
13 |
Indigofera heterantha |
Fabaceae |
Dicot |
|
14 |
Uraria neglecta |
Fabaceae |
Dicot |
|
15 |
Vicia augustifolia |
Fabaceae |
Dicot |
|
16 |
Vicia sativa |
Fabaceae |
Dicot |
|
17 |
Agrimonia pilosa |
Rosaceae |
Dicot |
|
18 |
Cotoneaster acuminate |
Rosaceae |
Dicot |
|
19 |
Fragria indica |
Rosaceae |
Dicot |
|
20 |
Potentillia nepalensis |
Rosaceae |
Dicot |
|
21 |
Potentillia fulgens |
Rosaceae |
Dicot |
|
22 |
Prinsepia utilis |
Rosaceae |
Dicot |
|
23 |
Prunus armeniaca |
Rosaceae |
Dicot |
|
24 |
Prunus persica |
Rosaceae |
Dicot |
|
25 |
Pyrus pashia |
Rosaceae |
Dicot |
|
26 |
Rosa moschata |
Rosaceae |
Dicot |
|
27 |
Rubus ellipticus |
Rosaceae |
Dicot |
|
28 |
Rubus paniculatus |
Rosaceae |
Dicot |
|
29 |
Woodfordia fructicosa |
Lythraceae |
Dicot |
|
30 |
Punica granatum |
Lythraceae |
Dicot |
|
31 |
Oenothera rosea |
Onagraceae |
Dicot |
|
32 |
Hedera nepalensis |
Araliaceae |
Dicot |
|
33 |
Cornus capitata |
Cornaceae |
Dicot |
|
34 |
Cornus oblonga |
Cornaceae |
Dicot |
|
35 |
Abelia triflora |
Linnaeaceae |
Dicot |
|
36 |
Leptodermis lanceolata |
Rubaceae |
Dicot |
|
37 |
Rubia cordifolia |
Rubaceae |
Dicot |
|
38 |
Artemisia parviflora |
Asteraceae |
Dicot |
|
39 |
Artemisia roxburghiana |
Asteraceae |
Dicot |
|
40 |
Bidens pilosa |
Asteraceae |
Dicot |
|
41 |
Anaphalis busua |
Asteraceae |
Dicot |
|
42 |
Cirsium verutum |
Asteraceae |
Dicot |
|
43 |
Eupatorium adenophorum |
Asteraceae |
Dicot |
|
44 |
Eupatorium riparium |
Asteraceae |
Dicot |
|
45 |
Inula cappa |
Asteraceae |
Dicot |
|
46 |
Tagetes minuta |
Asteraceae |
Dicot |
|
47 |
Taraxacum officinale |
Asteraceae |
Dicot |
|
48 |
Youngia japonica |
Asteraceae |
Dicot |
|
49 |
Jasminum grandiflorium |
Oleaceae |
Dicot |
|
50 |
Solanum xanthocarpum |
Solanaceae |
Dicot |
|
51 |
Solanum verbascifolim |
Solanaceae |
Dicot |
|
52 |
Adhatoda vasica |
Acanthaceae |
Dicot |
|
53 |
Barleria cristata |
Acanthaceae |
Dicot |
|
54 |
Lantana camara |
Verbinaceae |
Dicot |
|
55 |
Ajuga bracteosa |
Lamiaceae |
Dicot |
|
56 |
Ajuga parviflora |
Lamiaceae |
Dicot |
|
57 |
Salvia lanata |
Lamiaceae |
Dicot |
|
58 |
Origanum vulgare |
Lamiaceae |
Dicot |
|
59 |
Plantago major |
Plantaginaceae |
Dicot |
|
60 |
Cythula tomentosa |
Amaranthaceae |
Dicot |
|
61 |
Achyranthus aspera |
Amaranthaceae |
Dicot |
|
62 |
Fagopygon esculentum |
Polygonaceae |
Dicot |
|
63 |
Polygonum capitatum |
Polygonaceae |
Dicot |
|
64 |
Polygonum hydropiper |
Polygonaceae |
Dicot |
|
65 |
Polygonum barbatum |
Polygonaceae |
Dicot |
|
66 |
Rumex hastatus |
Polygonaceae |
Dicot |
|
67 |
Populus ciliate |
Salicaceae |
Dicot |
|
68 |
Salix lindleyana |
Salicaceae |
Dicot |
|
69 |
Asparagus recemosus |
Asparagaceae |
Dicot |
|
70 |
Cyperus rotundus |
Cyperaceae |
Monocot |
|
71 |
Cyanodon dactylon |
Poaceae |
Monocot |
|
72 |
Vitis himalayana |
Vitaceae |
Dicot |
|
73 |
Viola serpens |
Violaceae |
Dicot |
|
74 |
Paris polyphylla |
Melanthiaceae |
Monocot |
|
75 |
Coriaira nepalensis |
Coriariaceae |
Dicot |
DISCUSSION-
The
present study is conducted in the Doon valley located in the foothills of the
Himalayas. It is flourished by a variety of habitats and has unique topography
and climatic conditions. The Soil is generally medium loamy but its texture,
moisture, and pH generally varies from place to place and with time. The pH of
different habitats of Doon valley showed variation and at changing, pH
different types of angiospermic families have been observed and documented.
Mostly, the pH of soil was found to be acidic (5.5 - 6.8).
Due
to anthropogenic activities viz. liming, land pollution, increasing traffic,
urbanization, and industrialization etc pH of the soil is decreasing and seems,
it will approach to more acidic in near future. The relation
between soil and vegetation has been very important in natural woodland
ecosystems. Vegetation once established natural or by human interference
modifies soil developmental processes due to parent material, topography, and
climate change etc. and hence soil and vegetation relation are dynamic.
Sahastradhara
and Mussoorie are famous tourist places and mining areas, so highly disturbed
by anthropogenic activities. The invasion of Lantana camara, Parthenium
hysterophorus, Eupatorium adenophorum, and Ageratum conyzoides are abundant. The maximum dominance was found
to be of Parthenium hysterophorus with a mean percentage
cover of 15.5 followed by Lantana camera (mean cover 27.9%) [11],
but now it had been found that Lantana
had become a second threat to the western Himalayan forests due to its
environmental plasticity as it adjusts bin all types of habitats. In the
present study, we observed that the exotic species such as Lantana camara, Parthenium
hysterophorus, Ageratum conyzoides,
Eupatorium adenophorum, Murraya koenigii etc. have invaded the
large area of Doon valley and disturbed the local vegetation to a large extent.
The present findings are in agreed with the observations of Odum [7];
Dhyani and Joshi [12].
Variation
in soil pH influences the plant growth and is affected by rainfall patterns.
When pH reaches a value of 4 or bellows it limits the plant growth. Nutrient
availability and microbial activities are favored by a soil pH ranging from 5.5
– 8.5 as per the studies of Uchida and Hue [13]; Sheik et al. [14].
Major
factors influencing these changes are edaphic factors which include organic
matter, nutrient content, soil pH, and climatic factors which include weather
conditions weed competition etc. as per Arya [10]; Hassan
and Marwat [15]. Distribution pattern an important aspect of
ecological studies showed that all the three communities followed contagious
distribution as per Odum [7]. We found that herbs were the most dominant habit
followed by shrubs among all the plant forms follows to Sharma and Joshi [16].
Distribution
pattern is an important aspect of ecological studies and in present study we
find that all the three communities showed contagious distribution, and
contagious distribution is the most common type of distribution and it occurs
due to little but significant variation in environmental conditions, so in this
way our study showed similar results as of Dhayani et al. [17].
Therophytes were found to be in high
percentage and it is an indication of influences such as grazing [18],
and due to anthropogenic activities Manhas [19], which ensures the
further invasion of therophytes. It is experienced that vegetation in a stress
of biotic pressure gradually increases the percentage of therophytes. It is
pertinent to state that the composition of phanerophytes and therophytes is
close in this area, an increase in biotic pressure would change the biological
spectrum to therophytes and phanerophytes vegetation occurred.
Climate
change is a warning call and very well acknowledged threat today. As a result,
of climate change unexpected results occur, each species will respond in an
individual fashion according to its climate tolerance capacity and its ability
to disperse into a new location, and the species which will not adopt will extinct.
Rapid climate change favors the adoptability of those species that can tolerate
a wide range of climatic conditions. This adaptability is shown by many
invasive species in the study area as per the findings of Reshi [20].
Weeds such as Lantana camara, Parthenium hysterophorus, and Ageratum conyzoides have invaded and
altered the community structure of native flora of the study area as per the
findings of Rana [21]; Shigesada [22]. Climate change
enhances the dimensions of invasive species to occupy the new areas, by
disturbing the dynamic equilibrium maintaining them as per the observations of
Walther [23]; Holt et al.
[24].
Our findings goes parallel to them we also
find similar results of climate change and invasion of species such as Lantana camara, Ageratum conyzoides, Parthenium
hysterophorus, Eupatorium adenophorum and Murraya koenigii in
abundance. Species diversity and its
distribution along the altitudinal gradient has been a major subject of the
ecosystem. The diverse altitudinal range and rapid changes in altitudinal
gradient at very small distances and high endemism in Garhwal Himalaya make it
interesting for studies [25]. As we move from higher to lower
altitude biological diversity increases and vice versa on a mountain in a
terrestrial ecosystem as per the findings of Singh and Singh
[25].There are many broad ranges in the Garhwal Himalaya (<1500), High
altitude specialist genera having mere species such as Delphinium, Ranunculus,
Astragalus, Saxifraga, Sedum, Salix etc. are more abundant in
high altitudes as compared to adjacent low altitudes, as per the findings of
Joshi and Joshi [26]; Komar [27].
In
the present study observation is similar to [10], he stated that soil is mainly black grey and brown
in color, and in high elevation, it is of skeletal type. Soil temperature,
texture, and pH varied with elevations and proportion of sand 40.7% to 47.2%
increased somewhat with the increase in elevation. The Present study also
suggests that the soil type found in Mussoorie is generally medium loamy but
its composition, moisture, and pH generally varied from place to place. Higher
silt in Mussoorie was due to higher precipitation in the form of winter snow
above 1800 m Soil temperature in Mussoorie ranged from 40C – 160C,
while that of Sahastradhara was 15 – 190C. Soil organic matter
content tended to be higher in high altitude and increased with increasing
altitude respectively.
It
is rather difficult to compare the results of the present study with other literatures.
There is a need to make correlations between the availability of angiospermic
plants with physicochemical and biological variables. The results and findings
of the exercise are interpreted in the form of defined relationship between the
variables in question and definite risk to climate change in question.
CONCLUSIONS- Geographical
factors such as altitudinal variation is a major factor in species
distribution, as we move to high altitude there is a decrease in species
diversity, as up to an altitude of 5400 m plant growth is restricted. Although,
Physico-chemical properties of forest soils vary in space and time because of
variation in topography, climate, weathering process, vegetation cover and
microbial activities, and several other biotic and abiotic factors Our finding
observed that Soil temperature in Mussoorie ranged from 40C-160C,
while that of Sahastradhara was 150C-190C Sudhowala 150C
-250C, Soil organic matter content tended to be higher in high
altitude and increased with increasing altitude. In the present study also
stated that pH has a major effect on plant growth and distribution. It affects
the plant nutrient availability by controlling their chemical forms. The
optimum pH range for most of the plants is between 5.5-7, however many plants
are adapted to thrive at pH slightly above or below this range. But when pH
level falls to 4 it limits plant growth finally, we can say that ideal pH range
for plant growth is between 6.0-8 and pH below 5.6 is not suitable for proper
growth of the plant.
There
are several ways to rise and decrease soil pH, hence in the light of lack of
literature, it is rather difficult to compare the results of the present study
with others. There is a need to make correlations between the availability of
angiospermic plants with physicochemical and biological variables.
ACKNOWLEDGMENT-
Authors
wish to acknowledge the kind hospitality and valuable contributions of the
entire faculty in the Department of Botany, Alpine Institute Management
Technology, Dehradun during this field study. We are also highly grateful to
Botanical Survey of India (BSI), Dehradun, for identifying some of our plant
specimens.
CONTRIBUTION
OF AUTHORS
Dr.
Narendra Kumar- Data collection, Data analysis,
Research concept, Writing article, Research design,
Supervision, and Final approval.
Dr.
Kartik Uniyal- Materials collection, Critical review,
and Article editing.
Mr.
Zakir Nazir- Data collection and Literature search.
REFERENCES
1. Raina
AK, Jha MN, Pharasi SC. Sand mineralogy of some sodic soils of U.P. Ann. For,
2000; 8(2): 229–237.
2. Valdiya
KS. Stratigraphic scheme of sedimentary units of Kumaun lesser Himalaya. In;
Valdiya KS, Bhatia SB (Eds). Stratigraphy and correlations of Lesser Himalaya formations.
Hindustan publication corporation, Delhi, India, 1980; 7-48.
3. Hooker
JD, Thomson T. Introductory essay to Flora Indica, London: Pamplin. 1885.
4. Hooker
JD .A Sketch of Flora of British India. London, 1906.
5. Sharma
A, Singh H, Kumar N. Studies on traditional knowledge of medicinal flora and
its contribution to livelihood enhancement in the Doon valley, Uttrakhand,
India. I.J. Life. Sci. Scienti. Res., 2017; 3(2): 951-960.
6. Raunkiaer
C. The life form of plants and statistical geography. Claridon, Oxford, 1934;
p. 632.
7. Odum
EP. Fundamentals of Ecology, WB. Sounders and Co., Philadelphia, 1971; p. 557.
8. Schoenholtza
SH, Miegroetla HV, Burger JA. A review of physical properties as indicators of
forest soil quality: Challenges and opportunities, Forest ecology and
management, 2000; 335-356.
9. Raina
AK, Gupta MK. Soil and vegetation studies to parent material of Garhwal
Himalaya, Uttarakhand, India, Ann Fors, 2009; 17(1): 71-83.
10. Arya
MK .Assessment of physiochemical properties of soil along altitudinal gradients
in a protected forest in Kumaun Himalaya, India, Nat.&Sc, 2014; 12(2):
32-37.
11. Mandal
G. Analysis of vegetation dynamics and phytodiversity from three dry deciduous
forests of Doon valley western Himalaya, India, 2014; 7(3): 292-304.
12. Dhyani
S, Joshi SP. Angiospermic diversity of Karwapani freshwater swamp forest in
Doon valley Uttaranchal. Ind Fores, 2007; 1101-1108.
13. Uchida
R, Hue NV. Soil acidity and liming. Plant nutrient management in Hawaii soils,
approaches for tropical and subtropical agriculture and human resources,
University of Hawaii, 2000; p. 101-111.
14. Sheik
CS ,Mitchell TW, Rizvi FZ, Rehman Y, Faisal M, Hasmain H , Mclnerney MJ,
Krumholz LR. Exposure of soil microbial communities to chromium and arsenic
alters their diversity and structure, Int J of Mol Sci., 2012; 7(6); 933.
15. Hassan
G, Marwat KB. Integrated weed management in agriculture crops in national
workshop on technology for sustainable agriculture, 2001; 24-16.
16. Sharma
N, Joshi S. Comparative study of a Freshwater Swamp of Doon valley. The Journal
of American Science, 2008; 4(1): 7-10.
17. Dhayani
S, Saini N, Tiwari N. Community level floral diversity in Sahastrdhara forest
Dehradun Uttarakhand, Oct. Jour. Evn. Res, 2016; 4(3): 237-242.
18. Tiwari
S , Agrawal M, Manning WJ .Assessing the
impact of ambient ozone on growth and productivity of two cultivar of wheat in
India using three rates of application
of ethylene diurea. Environmental Pollution, 2005; 138: 153-160.
19. Manhas
RK, Kandwal MK, Dhyani S, Singh L, Joshi
SP. Effect of soil moisture on demographic dispersion, speciese association and
diversity of primary producer in a sub –tropical swamp forest. Indian Forester,
2007; 133(4): 547-560.
20. Reshi
ZA. Alien plant invasions in Himalayan Biodiversity Hot spot, Abstract book,
International workshop mountain biodiversity and impacts of climate change with
special reference to Himalayan biodiversity Hot spot, G.B Pant Institute of
Himalayan and Development, 2010; 104-109.
21. Rana
JC. Climate change impacting floral diversity and cropping patterns - A study
in the western Himalayan region, Abstract Book, International workshop mountain
biodiversity and impacts of climate change with special reference to Himalayan
biodiversity Hot spot, G.B Pant Institute of Himalayan and Development, 2010;
90-95.
22. Shigesada
N, Kawasaki K. Biological invasion. Theory and Practice. Oxford University
Press, Oxford, UK. 1997; p. 218.
23. Walther
GR. Ecology Tackling Geological complexity in climate impact research, Science
315; 2007; 606-607.
24. Holt
AC, Salkeled DJ, Fritz CL, Tucker JR, Gong P. Spatial analysis of plague in
California, niche modifying predictions of the current distribution and
potential responses to climate change. Int. J Health Geogr, 2009; 8: 38.
25. Singh
JS, Singh SP. Pattern of soil and vegetation and factors determining their
forms and hydrologic cycles in Nanda Devi Biosphere Reserve. Final technical
report submitted to Ministry of Environment and Forest New Delhi, 1992; 176.
26. Joshi
AK, Joshi PK. A rapid inventory of indicators of climate in the middle Himalaya,
2011; 100(6): 831-833.
27. Komar
PD. Beach process and sedimentation 2nd edition, Prentice-Hall upper saddle
river, NJ, 1998; 544.