1Dept. of Biotechnology, School of Sciences, Noida International University, GB Nagar, Greater Noida,
Uttar Pradesh, India
*Address for Correspondence: Dr. Archana Tiwari, Director, Dept. of Biotechnology, School of Sciences,
Noida International University, GB Nagar, Greater Noida, Uttar Pradesh, India
Received: 15 March 2017/Revised: 24 May 2017/Accepted: 21 June 2017
ABSTRACT-Brassica juncea, the Indian mustard supplies a big amount of edible oil demand in India. Biotic and Abiotic factors were responsible for serious reductions in B. juncea production in India. Several control measurements had been taken to prevent the losses in crop. Biochemical control, Biological control and Genetic control are some of the preventive methods used in this study to evaluate the yield loss in Indian Mustard. In India around 39 million hectares of land was under mustard cultivation with a production of 10 million tonnes. It was estimated that the demand for oilseed in India will be around 34 million tonnes by the year 2020. 41% of this demand (14 million tonnes) had to be met by mustard alone. Apart from its use as oil, it has got some medicinal properties too. Among the oilseed crops grown in India, mustard (Brassica sp.) was one the most important ones. In India around 26.11 million hectares of land was under mustard cultivation during the year 2009-2010 (4th advanced estimates released on 19.07.2010 by Ministry of Agriculture, Govt. of India). It was estimated that the demand for oilseed in India will be around 34 million tonnes by the year 2020. 41% of this demand 14 million tonnes must be met by mustard alone. The production of Indian mustard was severely affected by mustard aphids. In this review, we had studied some of the control method to avoid aphid infestation, which will severely affect the crop production.
Key-words- Biotic stress, Indian Mustard, Mustard Aphids, Genetic control
Indian mustard (Brassica juncea L.) was introduced to the northern part of India from China. It is a self-fertile annual. Presently about 25-30% of the total oilseed production in India is contributed by Brassica. Mustard seeds contain 6.2% Moisture, 24.6% Nitrogenous matter, 35.5% Fat, 8% Fibre and 5.3% Ash. The oil content of the crop is about 30 to 38%. Mustard oil contains about 60% monounsaturated fatty acid (MUFA) of which erucic acid oleic acid constitutes 42% and 12% respectively. It has 21% polyunsaturated fatty acid (PUFA) of which 6% is the omega-3 alpha-linolenic acid and 15% omega-6 linoleic acid and it has 12% saturated fats (SAFA). Indonesia is the highest producer of mustard seed with annual production of 21 million tonnes [1]. Apart from India, Brassica is also grown in China, Europe and Egypt. India produces bulk of mustard seed with annual productivity of 0.25 tonnes per ha and mustard is the second most important oilseed crop in India. It is a common field crop of Rajasthan, Uttar Pradesh, Haryana, Madhya Pradesh and Gujarat. Mustard oil was once the most popular cooking oil in northern India. In the second half of the 20th century the popularity of mustard oil receded due to the availability of mass-produced vegetable oils. Related to all other field crops mustard also suffers from various biotic stresses which utmost the final yield. Among the various biotic stresses experienced by mustard crop mustard aphid (Lipaphis erysimi L.) is the most important, [2] which causes severe yield losses all over the world. For centuries, peasants in rural India used the plant to add flavour to rice dishes. In that centuries, they called it the 'plant of long life', claiming it had powerful healing properties. Recent research into Indian Mustard's ancient medicinal claims has provided scientists with two remarkable findings.
First, the plant contains a robust mixture of anti-cancer ingredients that consists of vitamins, antioxidants and minerals with three times more calcium, potassium and iron than is develop in ordinary green-leafy vegetables. Second, it has a unique ability to absorb metals and minerals from the earth it grows in. For consideration into account, Russian agronomists have planted it near the site of the Chernobyl reactor to de-contaminate the ground from hazardous levels of lead and uranium.
Mustard is an annual herb cultivated mainly as oil seed crop. It is the second most important oilseed crop in India after Groundnut. Sometimes it is also used as vegetable or as fodder. Out of many species, 3 species are known for its condiment value. These are pale yel-low or white mustard (Brassica rapa), brown mustard (Brassica juncea) and black mustard (Brassica nigra). It has been introduced firstly to Northern India. The black mustard is endemic in the Southern Mediterranean region. Mustard prefers loamy or clayey loam soil. It is grown as Rabi crop in North India. It is raised during rainy season from July to November in South India.
CONTROL MEASURES
Aphid is one of the well-known and famous insect that is quite hard to constraint due to its elevated rate of reproduction. Aphid can be avoided to some extent if the crop is sown before 20th October. Applications of suggested dose of fertilizers, harming the affected plant tissues having aphid population at initial stages etc. are some cultural practices to overcome the aphid population. Biological control measures also offer some degree of crop protection. Ladybird beetles viz., Cocciniella septempunctata, Hippodamia variegata and cheilomones vicina are the most effcient pradators of the mustard aphid. Adult beetle may feed on an average of 10 to 15 adults per day. The lacewing, Chrysoperla carnea predates on the mustard aphid colony. Chemical control of mustard aphid includes spraying of insecticides below the ETL (Economic Threshold Level). Most commonly used chemicals are Imidacloprid 17.8% @ 0.25 ml/l, Thiamethoxam 25 WG @ 0.2g/l and Dimethoate 30EC @ 1 ml/l of water. But due to their nonspecific actions, beneficial insects like honey bees are adversely affected. More over chemical control becomes almost futile once aphids successfully establish and colonizes on the host plant due to their high rate of reproduction through parthenogenesis. Chemical insecticides cause environmental pollution which is a major concern regarding their use. Realizing these facts attention was given for generation of resistance in the host plant itself. Spray of Chemical insecticides can almost control the major insect pests and thus no more attempts have been made to breed varieties resistant to insects [3]. Traditional breeding approaches are not so victorious due to lack of re-sistance genes in the crossable gene pool [4]. Thus, genetic engineering technique accepts more importance to develop aphid resistance. Bacillus thuringiensis endotoxins have been studied extensively and proved to be effective against several insects falling under the groups of lepidoptera and coleoptera, which feed by chewing plant parts. But against this hemipteran sucking pest Bt toxin was found in-effective. Insecticidal properties of lectin genes have been utilized to develop insect resistance in many cases. Snowdrop lectin from Galanthus nivalis, commonly known as Galanthus nivalis agglutinin (GNA), had been found to be toxic to homopteran [5] and other major insect pests [6].
Wheat germ lectin, WGA, has been engineered into Brassica juncea and it was found that the transgenic plants became resistant to aphid infestation. Oryza cystatin encoding gene, OC-1 expressed in rapeseed affected the fe-cundity of the sap-sucking aphids although did not have much impact on insect survival [7]. Constitutive and phloem specific expression of Allium sativum leaf agglutinin (ASAL) in transgenic Indian mustard (Brassica juncea) has been found to be successful to combat aphid problem. So, efforts were started to develop aphid resistant mustard varieties by different methods which include Biological, chemical and Genetic control (Fig. 1). The transgenic Brassica plants were found to be tolerant to Lipaphis erysimi infestation.
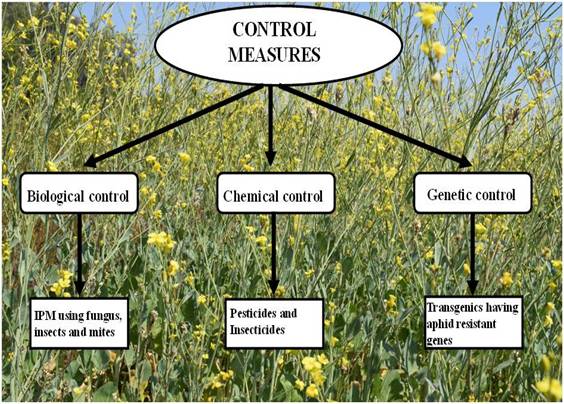
BIOLOGICAL CONTROL
In recent years, an effective and impressive body of knowledge on the biology and ecology of natural enemies of cereal aphids has been accumulated and as a result the only integrated pest management (IPM) activities relate to careful choice/application of pesticide and habitat modifications. Entomopathogenic fungi (Entomophthorales) are the most important microorganisms attacking cereal aphids. Their efficiency depends mainly on weather conditions during late spring and early summer: under favourable conditions, they can cause epizootics, and hence a fast crash of aphid populations [8-9]. The role of parasitoids is contradictory in the literature and often overestimated. Their advantage is often a good synchronization with their aphid hosts in time and space due to their close relation to the hosts, even during winter. However, only high parasitization rates during an early stage of the aphid infestation have a substantial impact. In addition, hyperparasitoids usually build up rapidly and limit parasitoid efficacy. In most years, rates of parasitism of cereal aphids during critical periods (e.g. the start of exponential population growth) are below 10%, much too low to influence aphid population dynamics [10-11]. Mass releases, habitat management, and increased diversity of the landscape can enhance locally the effectiveness of parasitoids [12-13]. Polyphagous, and particularly aphid specific, predators are more often the main factors in the natural mortality of cereal aphids than parasitoids. The predator community (in terms of species and guilds) in the cereal ecosystem is highly diverse, and its impact depends on the temporal and spatial synchronization, community composition, prey preferences, and predatory potential at given temperature conditions. Some species of polyphagous predators are spatially associated with cereal aphids through predation [14]. Under optimal conditions, polyphagous predators can reduce late aphid infesta-tions in cereals by up to 31% [15-19]. Carabids and most spiders show continuous, but relatively low, aphid consumption rates compared to aphid-specific predators [20-23]. Similarly, [24-25] showed (with seven potential generalist aphid predators) low or non-preference of epigeal predators for aphids and low food quality of cereal aphids. Thus, through early predation when alternative prey is scarce [23, 26] polyphagous predators can reduce the initial density of cereal aphids. Later, however, their voracity fails to keep up with increasing aphid densities, or they switch to more convenient prey [27]. The high voracity of aphid-specific predators [28-29] and their good synchronization in time and space with cereal aphids can greatly reduce the rate of population increase [30-35]. Syrphids and coccinellids particularly have a strong potential to regulate cereal aphid populations, showing both numerical and functional responses to their prey [36-39]. Despite a huge body of data, a proper assessment of the effects of single predator species on cereal aphid populations remains very difficult [40]. A simple addition of different predators with their varying aphid consumption rates is not possible. Therefore, it was devised the ‘predator unit’, whereby different predators can be assessed for their potential for aphid consumption [41]. For instance, a female Coccinella septempunctata receives the value 1.0, a male 0.88, and a green lacewing Chrysoperla carnea larva only 0.14. Multiplying these values with densities of each predator quantifies the impact of a predator community on their prey. Simulation models have been developed not only to describe the population dynamics of cereal aphids, but also to assess the impact of natural enemies [42-44]. Running such models with and without the presence of natural enemies provides a better understanding of regulation capacities [44].
Biological control may be defined as the use of one organism to reduce the population density of another organism. Defined biological controls of arthropods are defined as “the study and use of parasites, predators and pathogens for the regulation of host (pest) densities” [45]. Predators and parasitoids both are among the most important natural enemies of insects in many environments. The identification of parasitoids and predators found associated with aphids help to test their efficacy in suppressing their host in field conditions. The secondary symbiotic bacteria associated with aphids can confer immunity to parasitoid attack causing death of parasitoid eggs [46]. However, such resistance is suggested to be associated with a fecundity cost, as individual aphids with para-sitoid resistance produce fewer offspring. Interestingly, secondary symbiont mediated resistance of A. pisum to parasitism by Aphidius ervi is reported to increase when the aphid line is co-infected with both Serratia symbiotica and Hamiltonella defense compared to either of the singly infected lines [47-48]. Thus, a mechanism which can dissociate secondary symbionts from aphids will enhance the efficacy of biological control through parasitoid attack. Rhopalosiphum padi is a ubiquitous aphid vector of major cereal viruses like R. padi virus (RhPV). Upon infection with RhPV, aphid longevity and fitness decreases leading to reduction in colony populations. A recombinant baculovirus expression vector that expresses a full-length cDNA clone of RhPV was infectious in R. padi and was also transmitted efficiently between aphids [49]. Thus, the use of a baculovirus to express a small RNA virus RhPV opens avenues for large-scale production of small RNA virus bio-pesticides against aphids.
However, the contribution of parasitoids, predators, and fungal entomopathogens to suppression of aphid populations is less recognized by seed potato growers. It had been seen that unlikely the biological control agents could be effective given the intensive use of pesticides in seed potato production. However, the tremendous outbreaks that can be induced by insecticides when the aphids had developed resistance are indirect evidence of the importance of natural enemies [50-52].
CHEMICAL CONTROL
If aphid population exceeds through action thresholds, an insecticide treatment is recommended. In European countries, several efficient insecticides are registered for control of cereal aphids, both as BYDV vectors and as direct pests. The treatment decision is often based on simple economic considerations regarding both cost of application and amount of work involved. Hence, combined applications with fungicides before flowering are done. Of major concern are the possible side effects of drift on non-target organisms (both in the crop and on adjacent non-cereal areas) since, with cereals, large areas are treated simultaneously. Broad-spectrum insecticides like ?-cyhalothrin negatively affected several non-target organisms immediately after application [53]. However, such effects were often ephemeral, with rapid recovery and recolonization. Good agricultural practice (e.g. spraying at low wind speed using drift-reducing nozzles) can protect non-crop areas, even from highly toxic chemicals. Some European countries place restrictions on using pesticides in and near field margins to protect arthropod communities from spray drift [54]. Inherently more selective compounds like pirimicarb can be used; alternatively, some potent insecticides for cereal aphid control can be applied successfully at considerably reduced dose rates, especially if infestation is late and levels only marginally exceed the action threshold, as shown by [36-55] for ?-cyhalothrin and pirimicarb. Low dosage strategies are a very important element of IPM in cereals because of the considerably reduced side-effects on predators and parasitoids.
Although much of the insecticide used on potato is targeted against other pests, more than one-third of all applications in the USA are specifically for aphid control [56]. Insecticides are the only practical means of suppressing colonizing aphids on the crop, but are of inconsistent benefit in controlling virus spread. Among reported successes in controlling virus spread with insecticides (all crops and insect vectors), 94 of 119 cases involved persistent and semi-persistent viruses, [57] whereas most failures, 32 of 48 cases, involved non-persistent viruses. Viruliferous alatae are not killed quickly enough to prevent PVY transmission [58]. In contrast, spread of PLRV from within-field sources can be interrupted because of the extended post-acquisition latent period before an aphid can transmit [59-61]. Systemic insecticides applied at planting or plant emergence can reduce within-field spread of PLRV significantly [60-63]. Such timing gives the greatest benefit in locations where migrant aphids are rarely viruliferous. However, in the Pacific Northwest, PLRV infection rates can approach 100% if M. persicae is not controlled with insecticides [64]. Insecticide resistance often severely limits a grower’s choice of aphicides [65]. With M. persicae, this is a worldwide problem [66-67] and resistance has developed to all major insecticide classes except neonicotinoids [68-72] demonstrated that non-toxic mineral oils applied to plants substantially reduced PVY transmission. It is unclear why field control is generally inferior to that obtained in laboratory studies [73-75] but reasons probably include weathering of oil deposits [76] new plant growth between applications, and incomplete coverage.
The only and the most important method for controlling aphid infestation is the usage of high doses of agrochemicals but it is cost intensive in addition to being environmentally hazardous. The chemical pesticides include both contact and systemic insecticides. However, aphids are rarely killed with contact insecticides because they often infest the abaxial surface of leaves and feed directly from the phloem sap. Systemic insecticides which are absorbed by the plants are mainly used and popularly to control aphids, as it is ingested through phloem sap and kill the aphids irrespective of their shelter and feeding even if under the leaf. The prevalent agrochemicals used in the control of aphids include carbamates, organo-phosphates, pyrethroids, cyclodienes etc. group of pesticides [77-78]. Resistant populations of aphids develop against the usually sprayed organophosphate group of insecticides [79].
GENETIC CONTROL
Genetic resistance against any insect pest or pathogen can be achieved through conventional breeding ap-proaches by transferring resistance genes from sexually compatible germplasms. In developing aphid resistance, despite of substantial breeding efforts, resistant genotypes could not be bred mainly because of lack of resistance genes within the crossable gene pool [4]. To overcome the bottleneck of unavailable resistance source transgenic technology offers new avenues to explore resistance genes even from distant organisms.
Transgenic strategies expressing insecticidal Bacillus thuringiensis toxin, have been found to be effective against many insect pests belonging to the order Lepi-doptera and Coleoptera. But for sap sucking hemipteran aphids Bt toxin is ineffective. Engineering of other insecticidal proteins such as protease inhibitors, lectins, amylase inhibitors in crop cultivars also did not yield much resistance and as a result such researches remained confined to laboratory studies only. Therefore, it is imperative to look for new strategies by making use of new biological phenomenon to develop resistance against aphids. Transgenic cultivars were released in the USA that expressed the Leptinotarsa decemlineata (Colorado beetle) specific toxin Bacillus thuringiensis var. tenebrionis (Bt) combined with PLRV replicase [80] and other cultivars expressed Bt and PVY coat protein. This technology was far more effective than any presently used tactic, but these cultivars have been withdrawn because of concerns over a public backlash against genetically modified food.
Resistant and tolerant varieties can provide excellent control of aphid-vectored viruses [81-83]. Commercially available cucumber, zucchini, and yellow summer squash varieties [84] that have resistance or tolerance to one or more viruses, including genetically modified varieties that contain the coat protein genes of one or more viruses [82,85]. A new cantaloupe variety, ‘Hannah’s Choice’, developed in the USA by M. Jahn, a plant breeder at Cornell University, has resistance to WMV, PRSV-W, and ZYMV [86]. Recently, Harris Moran released the first pumpkin (Cucurbita pepo) variety, ‘Magician F1’, with tolerance to ZYMV. In Australia, a ‘Jarrahdale’ type pumpkin (C. maxima) has been released that is highly resistant to ZYMV, PRSV-W, and WMV [87]. Now, there are no commercially available virus-resistant or virus-tolerant varieties of watermelon in the USA. Resistance to A. gossypii and its transmission of viruses has been identified in muskmelon germplasm from India [88] and Korea [89]. However, examples of the practical use of this resistance are lacking. In Bangladesh, local genotypes of ash gourd (Benincasa hispida), also known as wax gourd or winter melon, are relatively resistant to A. gossypii [90]. The density of trichomes on leaves was negatively correlated with the number of aphids per leaf.
Many wild potato species are highly resistant to aphids [91]. Yet only limited use has been made of wild potato species in developing insect-resistant cultivars [91]. Various Agrobacterium-mediated transformations have produced potato lines expressing genes that confer pathogen-derived resistance to viruses. Transgenic lines have been developed that are highly resistant, but not immune, to infection by PLRV, PVY, and PVX [92]. While aphids can still acquire virus from low titre plants, efficiency of transmission is greatly reduced [64]. Transgenic cultivars were released in the USA that expressed the Leptinotarsa decemlineata (Colorado beetle) specific toxin Bacillus thuringiensis var. tenebrionis (Bt) combined with PLRV replicase [80] other cultivars expressed Bt and PVY coat protein. This technology of Agrobacterium-mediated transformation was far more effective than any presently used tactic, but these cultivars have been withdrawn because of concerns over a public backlash against genetically modified food crop.
Another most important genetic approach is to knock-down the genes responsible for aphid infestation is RNAi method. RNAi is known to be an effective way of gene silencing [93] in various organisms including plants [94] and insects [95]. The probability of using RNAi to kill the target insects by down regulating essential gene functions has been appreciated for several years [96]. One of need to explore RNAi technology for growing aphid resistance crop plants is to identify aphid genes which are significantly important for survival and colonization of the insect nymphs on host plants. cDNA sequences of genes or identified ESTs in mustard aphids are still limited in available databases. Additionally, the recognition of genes involved in early stage of infestation and colonization process by aphid insects will give the potential target for RNAi mediated down regulation and resistance. Targeted inactivation of indispensable aphid genes will lead to either retarded breeding cycle or induce lethality to aphids, which could be utilised as a strategy to breed aphid resistant crop cultivars. There are limited reports where RNAi has been strived to develop insect resistance.
BIOCHEMICAL RESPONSE DURING APHID INFESTATION
Plants respond through various morphological, biochemicals, and molecular mechanisms to counter the effects of aphid attack. The biochemical mechanisms of defense against the aphids are wide-ranging, highly dynamic, and are mediated both by direct and indirect defenses. The defensive compounds are either produced constitutively or in response to plant damage, and affect feeding, growth, and survival of aphids. In addition, plants also release volatile organic compounds that attract the natural enemies of the aphids. These strategies may act independently or in conjunction with each other. Although, the understanding of these defensive mechanisms is still restricted. The level of redox enzymes CAT, APX, and SOD, involved in ROS homeostasis in defense signaling, and several defense enzymes viz. POD, PPO, and PAL, remained high in infested plants [97]. Superoxide dismutase (SOD) protects the cell from oxidation due to reactive oxygen species (ROS) which interferes with the cellular metabolism [98-99].
CONCLUSIONS
In India, population is increasing day by day, to feed all these increased population we need to increase the yield per unit area as well as improve the resources used for efficiency of crops. 70% of Indian Mustard is cultivated in India is effected by Aphids. Losses in production due to biotic or abiotic factors are the major concerns. Traditionally, farmers are using chemicals to deal with aphids in Brassica fields. Moreover, in recent days, knowledge of plant-pathogen interaction, advanced breeding techniques including agricultural biotechnology are aided for resistance in response to aphid infestation in many crops like soybean, tomato, potato, brinjal, legumes, wheat, maize, melon, cotton, rice, barley, papaya and in rapeseed mustard. These approaches are much efficiently used in worldwide. QTLs is one of the most powerful approaches of molecular plant breeding used in genetic crop improvement. In QTL, important traits and genes associated with plant resistance to aphids are identified and incorporated into new cultivars using agriculture crop improvements tools. In this present review, we have tried to provide some of the innovative methods in response to aphid infestation. By considering the Genetic control, Chemical control and Biological control on this economically important crop, it is expected that the increased production trend can be achieved in a near future.
ACKNOWLEDGMENT
The authors are thankful to the Noida International University, India.
REFERENCES
- (FAOSTAT) Food and Agriculture Organization of the United Nations. 2009: http://www.fao.org/faostat/en/#home. Accessed on 4 May, 2017.
- Upadhyay, S. and Agrawal, R.K. Efficacy of different insecticides on incidence of mustard aphid L. erysimi on Indian mustard (Brassicae juncea) and its economic importance. Indian J. Agril. Sci., 1999; 63(8): 522-525.
- Rogers, C. E. Natural enemies of insect pests of sunflower: a world view. Texas Agric. Exp. Stu. Misc. Pub., 1980; 1456: 1-30.
- Yadava, J. S. et al. In: Oilseed Based Cropping Systems: Issues and Technologies, Project Directorate for Cropping Systems Res., Modipuram, Meerut, 1999; pp. 127-139.
- Gatehouse, A.M.R. et al. Insecticidal properties of plant lectins: their potential in plant protection. In: Pusztai A. & Bardocz S., eds. Lectins: biomedical perspectives. London: Taylor & Francis, 1995; pp. 35-58.
- Gatehouse, A.M.R. et al. Approaches to insect resistance using transgenic plants. Philos. Trans. R. Soc. London. Biol. Sci., 1993; 342: 279-286.
- Jouanin, L., Bonadé-Bottino, M., Girard, C., Morrot, G. and Giband, M. Transgenic plants for insert resistance. Plant Sci., 2003; 131: 1-11.
- Dedryver, C. A. Field pathogenesis of three species of Entomophthorales of cereal aphids in Western France. In: Aphid Antagonists (R. Cavalloro, ed.), 1982; 11-20.
- Ardisson, C., Pierre, J. S., Plantegenest, M., and Dedryver, C. A. Parameter estimation for a descriptive epizootiological model of the infection of a cereal aphid population by a fungal pathogen (Entomophthorale). Entomophaga, 1997; 42: 575–591.
- Borgemeister, C. and Setamou, M. et al. Biological control of the larger grain borer, Prostephanus truncates (Horn) by its predator Teretrius nigrescens in to go and benin. Biol. Control, 1992; 30: 241-55.
- Holler, C., Borgemeister, C., Haardt, H., and Powell, W. The relationship between primary parasitoids and hyperparasitoids of cereal aphids: An analysis of field data. J. Anim. Ecol., 1993; 62: 12–21.
- Levie, A., Legrand, M. A., Dogot, P., Pels, C., Baret, P. V. and Hance, T. Mass releases of Aphidius rhopalosiphi (Hymenoptera: Aphidiinae), and strip management to control of wheat aphids. Agr Ecosyst Environ., 2005; 105:17–21.
- Roschewitz, I., Hucker, M., Tscharntke, T. and Thies, C. The influence of landscape context and farming practices on parasitism of cereal aphids. Agricult. Ecosys. Environ., 2005; 108: 218–227.
- Winder, L., C. J. Alexander, J. M. Holland, W.O.C. Symondson, J. N. Perry, and C. Woolley. Predatory activity and spatial pattern: the response of generalist carabids to their aphid prey. J. Anim. Ecol., 2005; 74: 443- 454.
- Ekbom, B.S. and S. Wiktelius. Polyphagous arthropod predators in cereal crops in central Sweden, 1979-1982. J. Appl. Entomol., 1992; 99: 433-442.
- Winder, S. J., Allen, B. G., Fraser, E. D., Kang, H. M., Kargacin, G. J. and Walsh, M. P. Calponin phosphorylation in vitro and in intact muscle. Biochem. J., 1994; 296: 827–836.
- Holland, J. M. and Thomas, S. R. Quantifying the impact of polyphagous invertebrate predators in controlling cereal aphids and in preventing wheat yield and quality reductions. Ann. Appl. Biol., 1997; 131: 375-397.
- Holland. Insect vector interactions. Annu Rev Phytopathol., 1998; 45: 317–330.
- Ostman, O., B. Ekbom, and J. Bengtsson. Yield increase attributable to aphid predation by ground-living polyphagous natural enemies in spring barley in Sweden. Ecol. Econ., 2003; 45: 149-158.
- Sunderland, K. D., Crook, N. E., Stacey, D. L. and Fuller B. J. A study of feeding by polyphagous predators on cereal aphids using ELISA and gut dissection. J. Appl. Ecol., 1987; 24: 907–933.
- Triltsch, H. Food remains in the guts of Coccinella septempunctata (Coleoptera: Coccinellidae) adults and larvae. Eur. J. Entomol., 2001; 96: 355–364.
- Nienstedt, K. M. and Poehling, H. M. 15N-marked aphids for predation studies under field conditions. Entomol. Exp. Appl., 2004; 94: 319–323.
- Harwood, J. D., Sunderland, K. D. and Symondson, W. O. C. A quantitative assessment using monoclonal antibodies of the potential of the tetragnathids spider Pachygnatha degeeri to control aphids. Bull. Entomol. Res., 2005; 95: 161–167.
- Bilde, T. and Toft, S. The value of three aphid species as a food for a generalist predator. Physiol. Entomol., 2001; 26: 58–68.
- Toft, S. The quality of aphids as food for generalist predators: implications for natural control of aphids. Eur. J. Entomol., 2005; 102: 371-383.
- Madsen, M., S. Terkildsen, and S. Toft. Microcosm studies on control of aphids by generalist arthropod predators: Effects of alternative preys. Biocontrol, 2004; 49: 483-504.
- Sunderland, K. D. and G. P. Vickerman. Aphid feeding by some polyphagous predators in relation to aphid density in cereal Þelds. J. Appl. Ecol., 1980; 17: 389-396.
- Freier, B., Triltsch, H., Möwes, M., and Rappaport, V. The relative value of predators in natural control of cereal aphids and the use of predator units. Nachrichtenbl. Deut. Pflanzenschutzd., 1996; 49: 215-222.
- Tenhumberg, B. Estimating predatory efficiency of Episyrphus balteatus (Diptera: Syrphidae) in cereal fields. Environ. Ent., 1997; 24: 687-691.
- Chambers, R. J., and Adams, T. H. L. Quantification of the impact of hoverflies on cereal aphids in winter wheat: An analysis of field populations. J. Appl. Ecol., 1986; 23: 895-904.
- Poehling, H. M. Influence of cereal aphid control on specific predators in winter wheat (Homoptera: Aphididae). Entomol. Gen., 1988; 13: 163-174.
- Latteur, G. and R. Oger, Winter wheat aphids in Belgium: prognosis and dynamics of their populations. IOBC/WPRS Bulletin, 1991; 14(4): 13–34.
- Wetzel, T. and F. Schutte. Zur Schadens- und Bekiimpfungsschwelle der Getreideblattlaus (Macrosiphum [Sitobion] avenae [Fabr.]) an Winterweizen. Nachrichtenbl. Dtsch. Pflanzenschutzdienst (Berl.)., 1995; 40(12): 177-179.
- Poehling, H. M., Tenhumberg, B., and Groeger, U. Different pattern of cereal aphid population dynamics in northern (Hannover-Göttingen) and southern areas of West Germany. Bull. IOBC/WRPS, 1991; 14(4): 1–12.
- Elliott, N.C., Kieckhefer, R.W.and Beck, D. A. Adult coccinellid activity and predation on aphids in spring cereals. Biol. Control, 2000; 17: 218-226.
- Poehling, H. M. and Borgemeister, C. Abundance of coccinellids and syrphids in relation to cereal aphid density in winter wheat fields in northern Germany. IOBC/wprs Bull., 1989; 12(1): 99-107.
- Hemptinne, J. L., Dixon, A. F. G. and Coffin, J. Attack strategy of ladybird beetles (Coccinellidae): factors shaping their numerical response. Oecologia, 1992; 90: 238-245.
- Tenhumberg, B. and Poehling, H. M. Syrphids as natural enemies of cereal aphids in Germany: Aspects of their biology and efficacy in different years and regions. Agr. Ecosyst. and Environ., 1995; 52: 39-43.
- Freier, B., Triltsch, H., Hechenthaler, G. and Gosselke, U. How does a ladybird respond to aphids? IOBC/wprs Bull. 2001; 24(6): 49-58.
- Sunderland, T. C. et al. The Ethnobotany, Ecology and Natural Distribution of Yohimbe (Pausinystalia johimbe (K. schum.), an Evaluation of the Sustainability of Current Bark Harvesting Practices, and Recommendations for Domestication and Management. A Report Prepared for ICRAF, 1997.
- Freier, B., Möwes, M. and Triltsch, H. Beneficial thresholds for Coccinella 7-punctata L. (Col., Coccinellidae) as a predator of cereal aphids in winter wheat results of population investigations and computer simulations. J. Appl. Entomol., 1998; 122: 213-217.
- Carter, N., Dixon, A. F. G. and Rabbinge, R. Cereal aphid populations: biology, simulation and prediction. Centre for agricultural Publishing, Wageningen, 1982; pp. 91.
- Skirvin, D. J., Perry, J. N. and Harrington, R. A model describing the population dynamics of Sitobion avenae and Coccinella septempunctata. Ecol. Mod., 1997; 96: 29-39.
- Gosselke, U., Triltsch, H., Rossberg, D. and Freier, B. GETLAUS01 - the latest version of a model for simulating aphid population dynamics in dependence on antagonists in wheat. Ecol. Mod., 2001; 145: 143-157.
- DeBach, P. The scope of biological control. In Biological Control of Insect Pests and Weeds (P. DeBach, editor). Chapman and Hall Ltd., London, 1964; pp. 844.
- Gwynn, D. M., Callaghan, A., Gorham, J., Walters, K. F. A. and Fellowes, M. D. E. Resistance is costly: trade-offs between immunity, fecundity and survival in the pea aphid. Proceedings of the Royal Society B, London, 2005; 272: 1803–1808.
- Oliver, K. M., Russell, J. A., Moran, N. A. and Hunter, M. S. Facultative bacterial symbionts in aphids confer resistance to parasitic wasps. Proc. Natl Acad. Sci. USA, 2003; 100: 1803–1807.
- Oliver, K. M., Moran, N. A. and Hunter, M. S. Costs and benefits of a superinfection of facultative symbionts in aphids. Proc. R. Soc. B., 2006; 273: 1273–1280.
- Pal, N., Boyapalle, S., Beckett, R., Miller, W.A., Bonning, B.C., A baculovirus-expressed dicistrovirus that is infectious to aphids. J. Virol., 2007; 81(17): 9339–9345.
- French-Constant et al. Spontaneous loss and reselection of resistance in extremely resistant Myzus persicae (Sulzer) Pest. Biochem. Physiol., 1988; 30: 1–10.
- Harrington, R., Bartley, E., Riley, D.K., French-Constant, R.H.S. and Clark, S.J. Resurgence of insecticide resistant Myzus persicae on potatoes treated repeatedly with cypermethrin and mineral oil. Crop prot., 1989; 8(5): 340-348.
- Lagnaoui, A., Radcliffe, E.B. Potato fungicides interfere with entomopathogenic fungi impacting population dynamics of green peach aphid. Amr. Potato J., 1998; 7591: 19-25.
- Wick B. M. and Freier B. Long-term effects of an insecticide application on non-target arthropods in winter wheat – A field study over 2 seasons. Anzeiger fur Schadlingskunde, 2000; 73: 61–69.
- Cambell, C. L., Neher, D. A., K. N. Easterling and D. Fiscus. Comparison of nematode communities in agricultural soils of North Carolina and Nebraska. Ecol. Appl., 1998; 8(1): 213-223.
- Niehoff, B. and Hirche, H. J. Oogenesis and gonad maturation in the copepod Calanus finmarchicus and the prediction of egg production from preserved samples. Polar Biol., 1996; 16: 601–612.
- Guenther, A., B. Baugh, G. Brasseur, J. Greenberg, P. Harley, L. Klinger, D. Serca, and L. Vierling. Isoprene emission estimates and uncertainties for the Central African EXPRESSO study domain, J. Geophys. Res., 1999; 104(30): 625-639.
- Perring, T. M., Gruenhagen, N. M. and Farrar, C. A., Management of plant viral diseases through chemical control of insect vectors. Annu. Rev. Entomol., 1999; 44: 457-481.
- Shanks, C. H. and Chapman, R. K. The use of antiviral chemicals to protect plants against some viruses transmitted by aphids. Virology, 1965; 25: f13-7.
- Leonard, S.H. and Holbrook, F.R. Minimum acquisition and transmission times for potato leaf roll virus by the green peach aphid. Ann. Entomol. Soc. Am., 1978; 71: 493-495.
- Flanders, K. L., Hawkes, J. G., Radcliffe, E. B. and Lauer, F. I. Insect resistance in potatoes: sources, evolutionary relationships, morphological and chemical defenses, and ecogeographical associations. Euphytica, 1991; 61: 83–111.
- DiFonzo, C., D. Ragsdale, and E. Radcliffe. Potato leafroll virus spread in differentially resistant potato cultivars under varying aphid densities. Amr. J. Potato Res., 1995; 72: 119-132.
- Woodford, J. A. T. Virus transmission by aphids in potato crops. Neth. J. Plant Pathol., 1992; 98(2): 47–54.
- Boiteau, G. and Singh, R. P. Field assessment of imidaclo-prid to reduce the spread of PVYO and PLRV in potato. Amer. J. Potato Res., 1999; 76: 31–36.
- Thomas, P. E., Pike, K. S. and Reed, G. L. Role of green peach aphid flights in the epidemiology of potato leaf roll disease in the Colombian Basin. Plant Dis., 1997a; 81(11): 1311-1316.
- Radcliffe, E. B., Flanders, K. L., Ragsdale, D. W. and Moetzel, D. M. Pest management systems for potato insects. In D. Pimentel [ed.], CRC Handbook of Pest Management in Agriculture. 2nd ed. Vol. III. CRC Press, Boca Raton, Florida, 1991; pp. 587-621.
- Sawicki, R. M. Unusual response of DDT-resistant houseflies to carbinol analogues of DDT. Nature, 1978; 275: 443–444.
- Sawicki, R. M. and Rice, A. D. Response of susceptible and resistant peach-potato aphids Myzus persicae (Sulz.) to insecticides in leaf-dip bioassays. Pestic. Sci., 1983; 9: 513-516.
- Devonshire and Moores. A carboxylesterase with broad substrate specificity causes organophosphorus, carbamate and pyrethroid resistance in peach-potato aphids (Myzus persicae) Pest. Biochem. Phys., 1982; 18: 235–246.
- Devonshire et al. The evolution of insecticide resistance in the peach-potato aphid, Myzus persicae Philos. Trans. R. Soc. Lond. B Biol. Sci., (1998); 353: 1677–1684.
- Dewar, A. M., Haylock, L. A. and Garner, B. H. An appraisal of two insecticide seed treatments for sugar beet. Brit. sugar beet rev., 1998; 72(2-28): 30-32.
- Bradley, R. H. E., Wade, C. V. and Wood, F. A. Aphid transmission of potato virus Y inhibited by oils. Virology, 1962; 18: 327–328.
- Bradley, R. H. E., Moore, C. A. and Pond, D. D. Spread of Potato Virus Y Curtailed by Oil. Nature, 1966; 209:1370.
- Shands, W. A. Control of aphid-borne potato virus-Y in potatoes with oil-emulsions. Amer. Potato J., 1977; 54: 179–187.
- Boiteau, G. and Singh, R. P. Evaluation of mineral-oil sprays for reduction of virus Y spread in potatoes. Amer. Potato J., 1982; 59: 253–262.
- Bell, A.C. Use of oil and pyrethroid sprays to inhibit the spread of potato virus-Yn in the field. Crop Prot., 1989; 8: 37–39.
- Boiteau, G. and Wood, F.A. Persistence of mineral oil spray deposits on potato leaves. Amer. Potato J., 1982; 59: 55.
- Bahlai, C.A., Xue, Y., McCreary, C. M., Schaafsma, A. W. and Hallett, R. H. Choosing Organic Pesticides over Synthetic Pesticides May Not Effectively Mitigate Environmental risk in Soybeans. PlosONE, 2010; 5: e11250.
- Cameron, P. J., and Fletcher, J. D. Green peach aphid resistance management strategy. In: Pesticide resistance: prevention & management strategies. Editors N.A Martin, R.M. Beresford, K.C. Harrington. Hastings, NZ. New Zealand Plant Protection Society, 2005; 109-114.
- Gould, F. “Evolutionary Biology and Genetically Engineered Crops”, Bioscience, 1996; 38(1): 26-33.
- Thomas, P. E., Lawson, E. C., Zalewski, J. C., Reed, G. l. and Kaniewski, W. K. Extreme resistance to Potato leafroll virus in potato cv. Russet Burbank mediated by the viral replicase gene. Virus Res., 2000; 71(1-2): 49-62.
- Clough, G. H. and Hamm, P. B. Coat protein transgenic resistance to watermelon mosaic and zucchini yellows mosaic virus in squash and cantaloupe. Plant Dis., 1995; 79: 1107-1109.
- Tricoli, D. M., Carney, K. J., Russell, P. F., McMaster J. R., Groff, D. W., Hadden, K. C., Himmel, P. T., Hubbard, J. P., Boeshore, M. L., and Quemada, H. D. Field evaluation of transgenic squash containing single or multiple coat protein gene constructs for resistance to cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus. Bio/Technology, 1995; 13: 1458-1465.
- Walters, H. J. and P. Surin. Transmission and host range studies of Broad bean mottle virus. Plant Dis. Rep., 2004; 57: 833–836.
- Zitter, T. A., Hopkins, D. L., and Thomas, C. E. In: Compendium of Cucurbit Diseases. American Phytopathol. Soc., St Paul, MN, 2002; pp.87.
- Fuchs, M. and Gonsalves, D. Resistance of transgenic hybrid squash ZW-20 expressing the coat protein genes of zucchini yellow mosaic virus and watermelon mosaic virus 2 to mixed infections by both potyviruses. Biotechnol., 1995; 13: 1466-1473.
- Gordon, D. T. Maize dwarf mosaic. In: Lapierre H and Signoret PA [eds.], Viruses and Virus Diseases of Poaceae (Gramineae) INRA, Paris, 2004; pp. 644–649.
- Herrington, M. E., Byth, D. E., Teakle, D. S. and Brown, P. J. Inheritance of resistance to papaya ringspot virus type W in hybrids between Cucurbita ecuadorensis and C. maxima. Aust. J. Exp. Agr., 2004; 29: 253-259.
- Kishaba, A. N., G. W. Bohn and H. H. Toba. Resistance to Aphis gossypii in muskmelon. J. Econ. Entomol., 1971; 64: 935–937.
- Pitrat, M and H. Lecoq, Inheritance of resistance to cucumber mosaic virus transmission by Aphis gossypii in Cucumis melo. Phytopathology, 1980; 70: 985–961.
- Khan, et al. Impact of Bt-cotton on whitefly, Bemisia tabaci (Genn.) population Pak. J. Agric. Sci., 2000; 47(4): 327–332.
- Flanders, K. L., Arnone, S. and Radcliffe, E. B. The Potato: genetic resources and insect resistance. In: Clement SL, Quisenberry SS (Eds) Global Plant Genetic Resources for Insect-Resistant Crops, CRC Press, Boca Raton, FL, USA, 1999; pp. 207-239.
- Brown, C.R., Kwiatkowski, S., Martin, M.V. and Thomas, P.E. Eradication of PVY from potato clones through excision of meristems from in vitro, heat treated shoot tips. Amer. Potato J., 1995; 65: 633-638.
- Fire, A., Xu, S., Montgomery, M. K., Kostas, S. A., Driver, S. E. and Mello, C. C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature, 1998; 391: 806-810.
- Preuss, S., and Pikaard, C. S. Targeted gene silencing in plants using RNA interference. In: Engelke D, editor. RNA Interference (RNAi)~Nuts& Bolts of siRNA Technology, DNA Press, LLC, 2003; pp. 23–36.
- Possamai, J. S., Le Trionnaire, G., Bonhomme, J., Christophides, G. K., Rispe, C. and Tagu, D. Gene knockdown by RNAi in the pea aphid Acyrthosiphon pisum. BMC Biotechnol., 2007; 7-8.
- Price, D. R. G. and Gatehouse, J. A. RNAi-mediated crop protection against insects. Trends Biotechnol., 2008; 26: 393–400.
- Koramutla, M.K., Kaur, A., Negi, M., Venkatachalam, P., Bhattacharya, R. Elicitation of jasmonate-mediated host defense in Brassica juncea (L.) attenuates population growth of mustard aphid Lipaphis erysimi (Kalt.). Planta, 2014; 240: 177-194.
- McKerise, B. D. and Lesham, Y. Stress and stress coping in cultivated plants. Kluwer Academic Pub., 1994; Netherlands.
- Smirnoff, N. Tansley Review No. 52. The role of active oxygen in the response of plants to water deficit and desiccation. New Phytologist, 1993; 125(1): 27-58.
| How to cite this article: Jain V, Tiwari A: Innovative Approaches towards Aphid Resistance Prevention in Brassica Crops. Int. J. Life. Sci. Scienti. Res., 2017; 3(4):1230-1237. DOI:10.21276/ijlssr.2017.3.4.21 Source of Financial Support: Nil, Conflict of interest: Nil |