1Assistant Professor, Department of Biotechnology, Career College, Bhopal, India
2Assistant Professor, Department of Biochemistry, Career College, Bhopal, India
*Address for Correspondence: Tanuja Murab, Asst. Professor, Department of Biotechnology, Career College, BHEL,
Bhopal, (M.P.), India
Received: 09 March 2017/Revised: 15 May 2017/Accepted: 24 June 2017
ABSTRACT-Proteases is among the largest groups of industrial enzymes that also has the potential to contribute in the development of high value added products due to their characteristic nature that aids in digestion. Protease account for about 60% of the total worldwide sale of enzymes and is widely used in several industries ranging from silk industry, leather tanning, meat processing, organic fertilizers, diary and bioleaching. Bacteria produce a variety of proteolytic enzymes. Among them a major contributor of proteases producers is Bacillus subtilis. An attempt was made to formulate media using varied nitrogen sources to optimize media for maximum production of proteases. It was observed that media supplemented with soya meal as a nitrogen source had maximum biomass yield of 135 mg/ml while Tryptone supplemented media yielded 115.6 mg/ml and peptone supplemented media yielded only 101 mg/ml which was comparatively less than soya meal while the other nitrogen sources supplemented media were found to be poor in comparison to that supplemented by soya meal extract.
Key-words- Proteases, Bacillus subtilis, Optimize media, Soya meal extract
Bacillus subtilis is a gram negative rod shaped bacteria found commonly in soil. Bacillus subtillus is endospore forming bacteria and thus resistant to extreme physical conditions. It is an extremely valuable microbe as it produces a variety of proteolytic enzyme that is sable at varied physical conditions and is in high demand for commercial use. [1-3]
Proteases due to their huge application spectrum in various biotechnological processes have been the focus of intense research for many decades. Proteolytic enzymes are essential in various industrial sectors.[4-5] Proteases are quiet interesting as they can act on insoluble keratin substrates and on a variety of proteinacious substrates. [6] Although there are many microbial sources available for producing proteases, only a few are recognized as a commercial producers as they lack desired properties. [7] Proteases are one of the most important group of industrial enzymes and account for nearly 60% of the total enzyme sale The major uses of free proteases occur in leather industry [8], dry cleaning, detergents, meat processing, cheese making [9], silver recovery from photographic film, production of digestive and certain medical treatments of inflammation and virulent wounds. Due to the various demand and challenges faced search for better and efficient proteases, is a continuous practise. High cost of enzyme production is another major challenge for wide range of industrial applications of proteases and about 30-40% of production cost of industrial enzymes accounts for the cost of substrate/growth medium. [10] Exploring various cheap nitrogen sources for bulk production of industrial enzymes may play a pivotal role in reducing costs of proteases production through media optimization for culturing Bacillus subtilis.
MATERIALS AND METHODS
Screening and Isolation of proteases producing Bacillus subtilis- Soil samples were collected on December, 2015 from agricultural fields of Lalitpur district of Uttar Pradesh, India. Pure culture was obtained using Serial dilution method on NAM plates.[11] Six strains of Bacillus subtilis were identified namely BS-1, BS-2, BS-3, BS-4, BS-5 and BS-6 were isolated and identified based on cellular morphology, growth condition, and biochemical analysis. [12]
For protease screening, casein agar medium (g/l) (peptic digest of animal tissue, 5.0; beef extract, 1.5; yeast extract, 1.5; sodium chloride, 5.0, agar, 15, casein, 10 and 0. 0015% (w/v) BCG- Bromocresol Green Dye) was prepared and streaked with bacterial isolate (Bacillus subtilis) and in-cubated at 37°C for 48 hrs.[13] A zone of proteolysis was detected only on the casein agar plates of BS-5. Further analysis was conducted on BS-5 strain alone.
Optimization of media for maximum production of protease using different Nitrogen sources- The broth containing Bacillus subtilis was used for optimized production of protease enzyme consisted of varying nitrogen sources (urea, peptone, soya meal extract, ammonium sulphate, casein, KNO3, tryptone) 0.5 %, Glucose, 1% (w/v), yeast extract 0.55, KH2PO4 0.2%, Na2CO3 1%, MgSO4.7H2O 0.2%, and pH 8.0 at 140 rpm. [14-15] Optical density (OD) was taken using UV-visible spectroscopy at 660 nm at different time intervals and a graph was plotted (Fig. 1).
Calculation of dry weight of Bacillus subtilis (BS-5)-Dry weight was calculated for each broth (having dif-ferent Nitrogen sources) containing the cultures after 96 hrs.[3] One ml of cultures from each broth was transferred to centrifuge tubes of 1.5ml followed by centrifugation at 10,000 rpm for 15 minutes. The supernatant was discarded and the tubes containing the pellet were kept for air drying for overnight then the weight of cells was measured (Table 1).
RESULTS
The effect of different nitrogen sources (0.5g/l) on protease production by B. subtilis has been depicted in Fig. 1. On taking Optical density (OD) using UV-visible spectroscopy at 660 nm it was observed that the growth rate of B. subtilis decreased with time in case of urease (OD 0.261), casein (OD 0.330) and ammonia (OD 0.233) as nitrogen source after 72 hours with urease (OD 0.221), casein (OD 0.262) and ammonia (OD 0.233) after 96 hrs of incubation. While in case of Soya meal (OD 0.576), peptone (OD 0.555), tryptone (OD 0.383) and KNO3 (OD 0.231) the growth rate has still increased from 72 hrs to Soya meal (OD 0.592), peptone (OD 0.562), tryptone (OD 0.492) and KNO3 (OD 0.231) after 96 hours of incubation. Table 1. Depicts the dry cell weight of Bacillus subtilis after 96 hrs of incubation in media described above in material and method having various nitrogen supplements. It is observed that maximum dry weight is of soyameal extract and is organic in origin. It was also observed that the organic de-rivatives of nitrogen sources are more readily used by B. subtilis as compared to inorganic source of nitrogen.
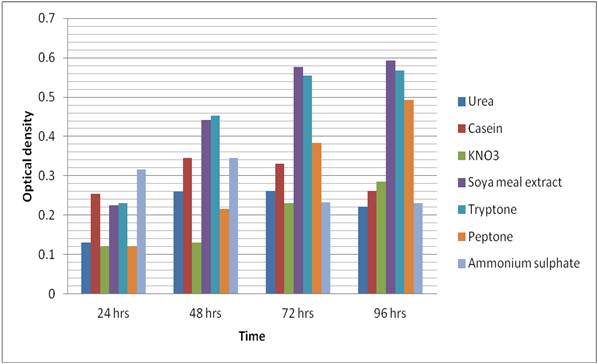
Table 1: Dry weight of Bacillus subtilis (BS-5)
DISCUSSION
six strains of Bacillus subtilis namely BS-1, BS-2, BS-3, BS-4, BS-5 and BS-6 were isolated and identified BS-5 was selected on the basis of its proteolytic activity. Bacillus subtilis is known to utilize crude substrates for protease production observed by Murab et al. [12]. The type and availability of nitrogenous precursor in the medium plays a crucial role in metabolism of extracellular enzyme as suggested by Kumar et al. [5] and Reddy et al. [17]. Although complex nitrogen sources were usually needed for proteases production, the requirement for a specific organic nitrogen supplement differs from organism to organism as observed by Lakshmi et al. [18]. The present study was undertaken with the objective to find out the effect of different nitrogen supplement for this various organic and inorganic nitrogen sources were investigated (urea, casein, KMNO3, soya meal extract, tryptone, peptone and ammonium sulphate) for growth of Bacillus subtilis (BS-5). Broth supplemented with various nitrogen sources were inoculated with Bacillus subtilis (BS-5) and incubated it was observed that soya meal extract yielded a significant increase in the growth rate as seen in (Fig. 1) followed by tryptone and peptone while other three supplements were found to be poor. On further analysis of the dry weight it was con-firmed that soya meal extract is best supplement for Bacillus subtilis BS-5 followed by supplements like tryptone and peptone. Urea, casein, KMNO3 and ammonium sulphate were found to be least yielding (Table 1).
CONCLUSIONS
The bacterial strain of Bacillius subtilis BS-5 in this study was previously isolated from soil sample. It was determined that soya meal extract is nitrogen supplement most productive for culturing Bacillus subtilis for large scale production in industries for extraction of proteases. Soya meal is the major by-product produces by soya bean industry while producing tofu, soya sauce and soya nuggets and thus a cheap source of nitrogen supplement for media formulation for Bacillus subtilis. Nitrogen source is one of the components i.e. needed to be optimized for further formulation of low cost media for growth of Bacillus subtilis without compromising the production capabilities of proteases. Further investigations are required to optimize media keeping in mind the variability of strain can change the outcome of the experiment.
ACKNOWLEDGMENT
We would like to acknowledge Career College, Bhopal for supporting and providing us with the facility and fund to aid us in our research work.
REFERENCES
- Das G and Prasad MP. Isolation, purification and mass production of protease enzyme from Bacillus subtilis. International Journal of Microb. 2010; 1(2):26-31.
- Imtiaz S, Mukhtar H and Haq I. African journal of microbiology research, Academic Journal. 2013; 7(16): 1558-1568.
- Rodarte MP, Dias DR, Vilela DM and Schwan RF. Proteolytic activities of bacteria, yeasts and filamentous fungi isolated from coffee fruit (Coffea arabica L.). Acta Scientiarum Agronomy. 2011; 33(3): 457-464.
- Khajuria V, Sharma K, Slathia P, Razdan K, Singh S and Bajaj BK. Production of a detergent-compatible alkaline protease from Bacillus cereus K-3. J. Mater. Environ. Sci. 2015; 6(8): 2028-2508.
- Kumar MM and Saranraj P.Commercial production and application of bacterial alkaline proteases: an overview.2015; 3(34):1-23.
- Agasthya AS, Sharma N, Mohan A, Mahal P. Isolation and Molecular Characterisation of Alkaline Protease Producing Bacillus thuringiensis. Cell Biochemistry and Biophysics. 2013; 66(1):45-51.
- Gupta R, Beeg QK, Khan S and Chauhan, B. An overview on fermentation, downstream processing and properties of microbial alkaline proteases. Appl. Microbiol. Biotechnol., 2002; 60(4): 381-395.
- Sathiya G. Production of protease from Bacillus Subtilis and Its Application In Leather Making Process. International Journal of Research in Biotechnology and Biochemistry. 2013; 3(1): 7-10.
- Koka R. and Weimer BC. Isolation and characterization of a protease from Pseudomonas fluorescens RO98. Journal of Applied Microbio. 2000; 89:280-288.
- El-Safey EM and Abdul-Raouf UM. Production, Purification and Charactrization of Protease Enzyme from Bacillus subtilis. International Conferences for Development and The Environment In The Arab World, Assiut Univ, 2004:14.
- Amin M, Rakhisi Z, Ahmady AZ. Isolation and identification of bacillus species from soil and evaluation of their antibacterial properties. Avicenna J Clin Microb Infec. 2014; 2(1):1-4.
- Murab T., Chandurkar P, Tripathi,N, Choudhary A. Characterization of Proteases Production by Varying Carbon Sources from Bacillus subtilis Isolated from Agriculture Soil of Lalitpur Dist. (U.P.) Int. J. Life. Sci. Scienti. Res.2016; 2(4): 454-456.
- Vijayaraghavan P, Vincent, SGP. A simple method for the detection of protease activity on agar plates using bromocresol green dye. Journal of Biochemical Tech 2013; 4(3): 628-630.
- Geethanjali S and Subhash A. Optimization of protease production byBacillus subtilus isolated from mid gut of fresh water fish Labeo Rohita. World journal of fish and marine science.2011; 3(1):88-95.
- Nisha NS, Divakaran J. Optimization Parameters for Alkaline protease Production using Bacterial isolates from different coastal regions of Tamil Nadu, India. International Journal of Curr. Microbiol. App. Sci. 2014; 3(8) 500-505.
- Lakshmi BKM, Ratna Sri PV, Devi KA, Hemalatha KPJ. Media optimization of protease production by Bacillus licheniformis and partial characterization of Alkaline protease. Int. J. Curr. Microbiol. App. Sci. 2014; 3(5):650-659.
- Reddy LVA, Wee YJ, Yun JS, Ryu HW. Optimization of alkaline protease production by batch culture of Bacillus sp. RKY3 through Plackett-Burman and response surface methodological approaches. Bioresource Technology. 2007; 99 (2008) 2242–2249.
- Gerday C, Chessa JP, Petrescu I, Bentahira M, Beeumen JV. Purification, physicochemical characterization and sequence of a heat labile alkaline metalloprotease isolated from a psychrophilic Pseudomonas species. Biochimical Biophysical Acta. 2000; 1479(1-2):265-274.
| How to cite this article: Murab T, Chandurkar P, Tripathi N, Choudhary A, Saini S, Gujar N: Characterization of Proteases Production by Varying Nitrogen Sources from Bacillus subtilis Isolated from Agriculture Soil of Lalitpur Dist. UP. Int. J. Life. Sci. Scienti. Res., 2017; 3(4):1215-1217. DOI:10.21276/ijlssr.2017.3.4.18 Source of Financial Support: Nil, Conflict of interest: Nil |