1Professor and HOD, Department of Human Genetics, Punjabi University, Patiala, Punjab, India
2M.Sc student, Department of Human Genetics, Punjabi University, Patiala, Punjab, India
3Research scholar, Department of Human Genetics, Punjabi University, Patiala, Punjab, India
*Address for Correspondence: Ms. Nisha Gautam, Research scholar, Department of Human Genetics, Punjabi
University, Patiala (147002), Punjab, India
Received: 08 March 2017/Revised: 23 May 2017/Accepted: 25 June 2017
ABSTRACT-The study was conducted to assess DNA damage in peripheral blood lymphocytes of cancer patients put on radiotherapy, medical workers occupationally exposed to ionizing radiations and control group of normal healthy individuals. The blood samples were collected from 20 cancer patients undergoing radiotherapy in Government Rajindra hospital, Patiala, 16 medical workers from Radiology and Radiotherapy department of Government Rajindra hospital, Mata Kaushalya hospital, T.B. hospital, Patiala and 10 normal healthy individuals from Punjabi University, Patiala, India. The DNA damage was evaluated by using alkaline COMET assay, the damage was assessed from two COMET parameters i.e. mean COMET tail length and frequency of cells showing migration. It was found that all the cancer patients undergoing radiotherapy as well as the medical workers, who were occupationally exposed to ionizing radiations for variable period of time showed DNA damage, whereas none of the control subjects showed any damage. The comparison of DNA damage between the cancer patients and medical workers revealed highly significant differences. On the basis of results obtained, it could be said that the exposure to acute high doses of radiations cause greater DNA damage in comparison to chronic low doses of radiations.
Key-words- Single cell gel electrophoresis, Micronuclei, Thermoluminescent dosimeter, Aberration
The maintenance of DNA from one generation to the next is one of the primary goals of our biological sys-tems. Any gross change in its structure, base sequence is referred as DNA damage, which can pose serious biological changes in the body. Detection of DNA damage in individual cells was first reported in 1978 [1]. The damage was quantified by measuring the ratio of green fluorescence (indicating double strand DNA) to red fluorescence (indicating single strand DNA). The evaluation of the DNA damage plays an important role in genotoxic studies. The techniques utilized to detect DNA damage in humans have involved cytogenetic evaluation of chromosomal aberrations, sister chromatid exchanges in mitogen stimulated peripheral blood lymphocytes, Micronuclei assay, immuno-assays and single cell gel electrophoresis (COMET assay).
The technique known as SCGE was developed in 1984 by the introduction of micro electrophoresis, which was later modified in 1988 [1]. Single cell gel electrophoresis is one of the most commonly used assays for the population biomonitoring to identify the DNA instability. It is a simple, rapid, visual and sensitive technique to detect DNA single strand breaks, double strand breaks and alkali labile sites/damage. This technique was based on the principle that DNA is negatively charged and when the electric field is applied, it moves towards the anode. Singh modified the technique of electrophoresis originally given by Ostling [2-4]. They replaced the neutral buffer with alkaline buffer (pH>13) during electrophoresis in order to detect the presence of single strand breaks and alkali labile damage in individual cells. The technique has been used to evaluate both DNA damage and repair in the cancer cell lines, cancer patients undergoing radiotherapy/chemotherapy, occupationally exposed workers etc. Though radiotherapy is a powerful weapon against cancer and has great significance but the radiations also produce marked toxicity at the cancer sites, ultimately resulting in the DNA damage leading to second cancer. Several studies used COMET assay to evaluate DNA damage in cancer cell lines [5-7] in cancer patients undergoing radiotherapy [6,8] and in workers occupationally exposed to radiations. Majority of such studies have indicated radiation induced DNA damage in various cancer cell lines and in peripheral blood lymphocytes of cancer patients put on radiotherapy [9-10]. An inverse correlation was found between cell survival and COMET tail length [11]. Even when the peripheral blood lymphocytes of healthy individuals were exposed to variable doses of X-rays or ?-radiations, comets were formed, indicating DNA damage which was correlated with extent of the dose. Higher doses induced greater DNA damage [12]. Work done on DNA repair revealed quick repair of single strand breaks (SSBs) in comparison to double strand breaks (DSBs) [13]. Significant increase in the damage was reported in the workers who were occupationally exposed to variable doses of radia-tions[14-18].
MATERIALS AND METHODS
Subjects- Duration of the study was one year. The study was conducted on 20 cancer patients undergoing radiotherapy (group A), 16 workers occupationally exposed to radiations (group B) and 10 healthy subjects (group C). Group A subjects were selected from Government Rajindra hoapital, Patiala. Only those pa-tients who had undergone at least 5 cycles of radio-therapy were selected for the study. Group B workers were selected from T.B. hospital and Government Rajindra hospital, Patiala, workers working on X-ray machine or handling machines for at least 5 years. All the workers wore individual TLD (Thermoluminescent dosimeter) badges during their work. The TLD badges were replaced after every 3 months and were sent to BARK, Bombay for estimating radiation exposures. The accumulative dosimeter readings of the exposed workers during the year prior to the study were recorded from the hospital records.
Blood Samples- About 0.5 ml of whole blood was collected from fingertip using a sterilized lancet from selected subjects. Blood samples were collected in eppendorf tubes containing one drop of EDTA solution. The tubes were numbered and stored in refrigerator till use.
COMET Assay- The alkaline COMET assay was performed on lymphocytes under alkaline conditions according to Singh in 1988 with slight modifications [19]. An aliquot of 40 µl of whole blood was used to assess the DNA damage. Slides were prepared in duplicates per sample. Slides were covered with normal melting point agarose (NMPA) and dries for further use. For the second layer 25 µl of whole blood was added to 75 µl of 0.5% low melting point agarose (LMPA) and pipetted out on the precoated slides. The slides were covered with coverslips for the proper spreading of the cells. Further the slides were allowed to gel in the 4şC for 30 minutes. The coverslips were removed and third layer of 110 µl of LMPA was pipetted out on the slides and allowed to gel at 4şC for 30 minutes. After third layering, the uncovered slides were immersed in freshly prepared chilled lysing solution and refrigerated for 2 hours. Slides were then placed in alkaline electrophoretic buffer (E-buffer, pH=13) for 20 minutes to allow unwinding of the DNA. Electrophoresis was conducted for 25 minutes at 25V adjusted to 300mA by raising or lowering the buffer level in the tank. Slides were then drained, placed on a tray and washed thrice with neutralizing buffer (N-buffer, pH =7.5). Finally all the slides were stained with silver nitrate in the presence of dim light so as to minimize artefactual DNA damage, further the slides were dried and viewed under a microscope. Analysis was performed by using 10x and 40x objectives Zeiss microscope.
A total of 100 individual cells were screened per sample (50 cells per slide). An undamaged cell resembles an intact nucleus without a tail and a damaged cell has the appearance of a comet. The length of the DNA migrated in the comet tail, which is an estimate of DNA damage was measured by using an ocular micrometer. Quantification of DNA damage for each cell was cal-culated as given in the formula:
T/N index = Mean tail length/ Head diameter
The significant differences between the different groups (cancer patients and medical workers/controls), (medical workers/cancer patients) and (Alcoholic cancer patients/Non alcoholic cancer patients) for comet parameters were analyzed by calculating mean, standard deviation and student's t-test.
RESULTS
The detailed history of cancer patients (N=20), medical workers (N=16) and controls (N=10) is shown in Table 1. The DNA damage of 46 subjects (20 radiation induced cancer patients, 16 occupationally radiation exposed medical workers and 10 controls) was evaluated by using comet assay analysis as shown in Fig 1. The damage was assessed by considering two comet parameters i.e., frequency of cells showing migration and mean comet tail length.
Table 1: Medical history of cancer patients undergoing radiotherapy
* Indicate (M/F=Male/Female, S/NS=Smoker/Non-smoker, A/NA=Alcoholic/Non-alcoholic, cGy-Gray, MTL= Mean comet tail length, µm-micrometer)
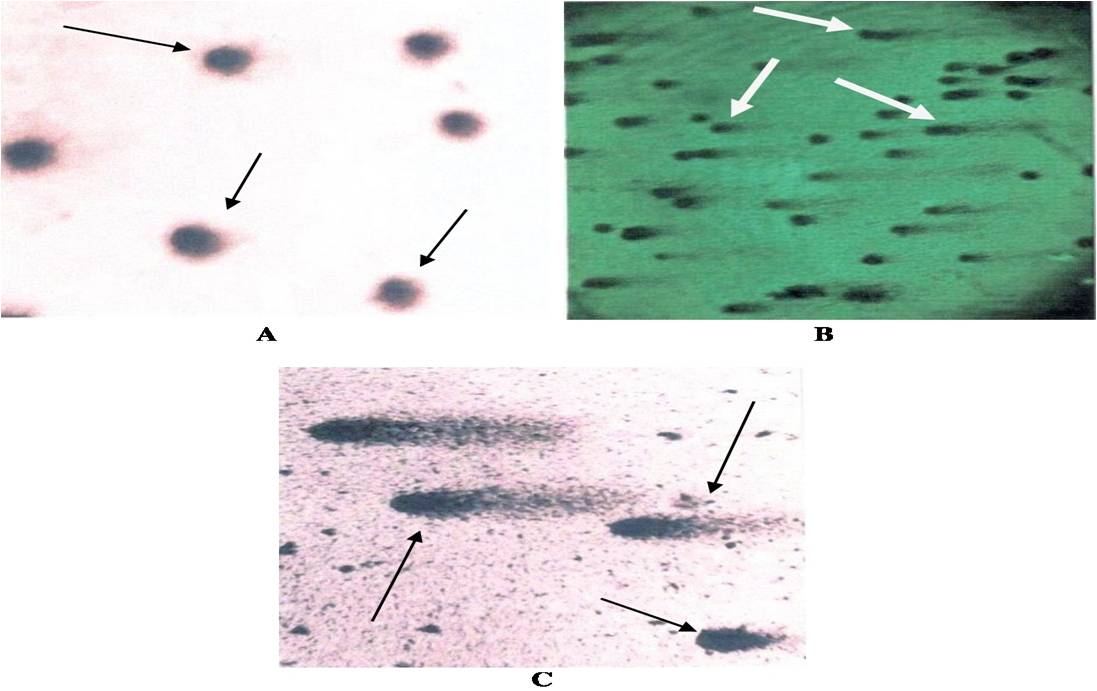
and (C= cancer patients) highly damaged DNA
Table 2: DNA damage in cancer patients, medical workers and in controls
Comparison of cancer patients with medical workers showed highly significant (P<0.01) differences in both the comet parameters (Table 2). Non-significant differences (P>0.05) were found, when comet variables were compared in relation to variable doses of radiation exposure in cancer patients as shown in Table 3. Life style factors including age and smoking also revealed non-significant effect on the comet variables whereas the alcohol intake showed significant effect on the mean comet tail length.
Table 3: DNA damage in relation to the exposure of variable doses of radiation
The comet tail length was significantly higher in alcoholic cancer patients (72.49± 1.95 v/s 65.33±5.54, P<0.05) compared to non-alcoholic cancer patients (Table 4).
Table 4: DNA damage in relation to age, smoking habit and alcohol consumption
The type of radiations, duration of radiation exposure and as well as other confounding factors such as age, smoking and alcohol consumption did not reveal any significant effect on the comet variables in the medical workers who were occupationally exposed to ionizing radiations.
DISCUSSION
The comet assay is considered as a well known bi-omarker for assessing DNA damage due to radiation exposure. Among the other biological parameters that have been used in this area, cytogenetic damage in peripheral blood lymphocytes is known as most promising [20]. It includes chromosomal aberrations, micronucleus test and sister chromatid exchanges. Frequencies of chromosome aberrations and micronuclei are known to be increased among individuals occupationally exposed to ionizing radiations [14,21]. It is well established fact that ionizing radiations can induce DNA damage directly by depositing energy in the cells or indirectly as a result of free radical formation and oxidative stress. The physiochemical interactions between ionizing radiations and DNA produce a variety of DNA lesions such as damage to nucleotide bases, DNA-DNA and DNA-protein crosslinks and alkali labile sites as well as single strand breaks and double strand breaks. Misrepaired double strand breaks are considered to be the principle lesion of importance in the induction of both, chromosomal aberrations and gene mutations [22-23]. It is widely accepted that lesions induced by ionizing radiations in DNA can be detected by using alkaline comet assay [14,16].
In the present study, the comet assay was used to find out the effect of acute high dose and chronic low dose of ionizing radiations on the peripheral blood lymphocytes. For this purpose, DNA damage was studied in 20 cancer patients put on radiotherapy, 16 medical workers occupationally exposed to X-rays and ?-rays and 10 normal healthy controls. All the cancer patients and medical workers showed DNA damage but the extent of damage was significantly higher among the cancer patients. Similar findings have been reported by many earlier studies [21,24-26] except one study in which the authors have reported negative results [27]. The comet tail length was surprisingly very low in both, treated (radiotherapy) and untreated cancer patients (ranging between 0.91 to 3.76). The combined data of 20 cancer patients have shown DNA damage in 62% to 100 % cells, with a mean value of 87.6 ±9.76 whereas not even a single cell showing DNA damage was found in control samples. The mean comet tail length (MTL) in cancer patients ranged from 54.46 microns to 76.93 microns. The extensive DNA damage found could be attributed to the radiation therapy, though the possibility of the disease (cancer) itself causing DNA damage cannot be ruled out, as many previous studies have found significantly higher DNA damage in untreated cancer patients compared to controls [25,27-29]. The frequency of damaged cells showed non-significant increase with a corresponding increase in the level of radiation exposure. Earlier studies, both in vitro and in vivo, have indicated a significant dose dependent increase in the DNA damage [17-18,30]. Response to radio-therapy was quite similar in all the cancer patients irrespective of the fact that these patients were having different types of cancers and were ex-posed to variable doses of ?-radiations. All the exposed medical workers, showed DNA damage in their peripheral blood lymphocytes and none of the control subjects showed any damage. Frequency of cells showing migration varied from 25 % to 63% and mean comet tail length varied between 23.75% and 55.77 %. An earlier study, conducted on the exposed medical workers [16,31] revealed intra-individual heterogeneity in the level of DNA breakage. No correlation between DNA damage and dose of the radiation was reported.
None of the probable confounding factors such as age, smoking habits, alcohol intake, duration of exposure and type of exposure showed any effect on DNA dam-age. This is in agreement with previous findings [14,32]. However, some authors [33-34] had reported an associa-tion between smoking and increased DNA migration during comet assay.
CONCLUSIONS
In this conclusion, this was a preliminary work to assess the radiation induced DNA damage in human peripheral blood lymphocytes of occupationally exposed medical workers and cancer patients. It was found that medical workers and cancer patients have shown radiation induced DNA damage. But the level of DNA damage was found to be more prominent in cancer patients in comparison to medical workers. The study was statistically found to be significant and none of the cell from control samples have showed any kind of DNA damage. The results revealed that radiation may cause extensive DNA damage which could further increase the recurrence risk of having second cancer. The present study may contribute to new insight of implication of less damaging strategies for treatment of cancer such as precision therapy which is an approach to cancer care that allows doctors to select treatments that are most likely to help patients based on a genetic understanding of their disease.
REFERENCES
- Barbara S, Eliska G, Kusenda B, Slaninova M, Vlcek D, Dusinska M. The Comet assay and the troubles with its application in the green alga Chlamydomonas reinhardtii. Czech Phycology, Olomouc, 2004; 4: 163-174.
- Ostling O, Johanson K J. Microelectrophoretic study of radiation–induced DNA damages in individual mammalian cells. Biochem, Biophys Res, 1984; 123 (1): 291–298.
- Singh N. P, Tice R. Mccoy M. T, Schneider E. L. A simple technique for quantition of lox levels of DNA damage in individual cells. Exp Cell Res, 1988; 175 (1): 184–191.
- Olive P. L, Banath, J P, Evans H. H. Cell killing and DNA damage by etoposide in Chinese hamster V79 monolayers and spheroids: influence of growth kinetics, growth environment and DNA packaging. Br J Cancer, 1993; 67 (3): 522–30.
- Anderson D, Yu TW, Phillips BJ, Schmezer P. The effects of various antioxidants and other modifying agents on oxygen-radical-generated DNA damage in human lymphocytes in the comet assay. Mutat Res, 1994; 307: 261-271.
- Bao S, Qiulian Wu , Roger E, McLendon , Yueling H, Qing S , Anita B. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response . Nature Protoc ols, 2006; 444: 756-760.
- Peggy L, Olive, Judit P, Banáth. The comet assay: a method to measure DNA damage in individual cells. Nature Protocols, 2006; 1: 23 - 29.
- Morris ID. Sperm DNA damage and cancer treatment. Int J Androl, 2002; 25: 255-261.
- Tice RR, Agurell E, Anderson D, Burlinson B, Hartmann A, Kobayashi H, Miyamae Y, Rojas E, Ryu JC, Sasaki YF. Single cell gel/Comet assay: guidelines for in vitro and in vivo genetic toxicology testing. Environ Mol Mutagen, 2000; 35: 206–221.
- Popanda O, Marquardt JU, Chang-Claude J, Schmezer P. Genetic variation in normal tissue toxicity induced by ionizing radiation. Mut Res, 2008; 10: 34-40.
- Kelly MR, Xu J, Alexander KE, Loo G. Disparate effects of similar phenolic phytochemicals as inhibitors of oxidative damage to cellular DNA. Mutat Res, 2001; 485: 309–318.
- Rydbe, Cooper B, Cooper PK, Holley WR, Chatterjee A B. Dose-dependent misrejoining of radiation-induced DNA double-strand breaks in human fibroblasts: experimental and theoretical study for hig- and low-LET radiation. Radiat res, 2005; 163(5):526-34.
- Yoo S, Dynan WS. Geometry of a complex formed by double strand break repair proteins at a single DNA end: recruitment of DNA-PKcs induces inward translocation of Ku protein. Nucleic Acids Res, 1999; 27(24): 4679–4686.
- Maluf SW, Passos DF, Bacelar A, Speit G, Erdtmann B. Assessment of DNA damage in lymphocytes of workers exposed to X-radiation using the micronucleus test and the comet assay. Environ Mol Mutagen, 2001;38: 311-315.
- Touil N, Aka PV, Buchet JP, Thierens H, Kirsch-Volders M. Assessment of genotoxic effects related to chronic low level exposure to ionizing radiation using biomarkers for DNA damage and repair. Mutagenesis, 2002; 17 (3): 223-232.
- Vera GV, Nevenka K. The alkaline Comet assay as biomarker in assessment of DNA damage in medical personnel occupationally exposed to ionizing radiation. Mutagenesis, 2003; 18(3): 265-271.
- Cuza B, Teodor MC, Mihaela Oprea, L, Gorgan D, Mihai-lescu. In vitro study of radiation-induced DNA damage. Romanian Res, 2014; 66: 16–21.
- Soto MG, Castro MR, Gerardo EC, Norma PC. Evaluation of Hypoglycemic and Genotoxic Effect of Polyphenolic Bark Extract from Quercus sideroxyla. Evid Based Complement Alternat Med, 2016; 10: 1-7.
- Ahuja YR, Saran R. Potential of Single Cell Gel Electrophoresis Assay (Comet Assay) in Heavy Ion Radiation Biology. IJHG, 2001;1 (2): 151-156.
- Stetter J, Lieb F. Innovation in Crop Protection: Trends in Research. Angew Chem Int Ed Engl, 2000; 39 (10): 1724-1744.
- Vera GV, Nevenka K. The comet assay – a new technique for detection of DNA damage in genetic toxicology studies and human biomonitoring. Period Biol,1998; 100: 361-366.
- Little M, Carman G, Donaldson E. Novel WT1 exon 9 mutation (D396Y) in a patient with early onset Denys Drash syndrome. Hum Mutat, 2000; 15(4): 389-394.
- Gloss LM, Placek BJ. The effect of salts on the stability of the H2A–H2B histone dimer. Biochemistry, 2002; 41 (50): 14951-9.
- Adiga S K, Jagetia GC. Effect of teniposide (VM-26) on the cell survival, micronuclei-induction and lactate dehydrogenase activity on V79 cells. Toxicology, 1999; 138: 29-41.
- Palyvoda O , Joanna P, Andrzej W, Joanna RW. DNA Damage and Repair in Lymphocytes of Normal Individuals and Cancer Patients: Studies by the Comet Assay and Micronucleus Tests. Acta Biochim Pol, 2003; 50 (1): 181-190.
- Cao N, Jia KL, Qing YR, Liu H, Ji W, Li B, Peiquan Z, Zeng L. A potential role for protein palmitoylation and zDHHC16 in DNA damage response. Molecular Biol, 2016; 11: 1-11.
- Jialin L, Jiliang H, Lifen J, Wei R, Hongping D. Measuring genetic damage in cancer patients during radiotherapy with three end genetic points. Mutagenesi,. 2004; 19(6): 457-464.
- Khopjar N, Vera VJ. Application of the alkaline comet assay in human biomonitoring for genotoxicity: A study of Croatian medical personnel handling antineoplastic drugs. Mutagenesis, 2001; 16: 71-78.
- Grossman SR, Deato ME, Brignone C, Chan HM, Kung AL, Tagami H, Nakatani Y, Livingston DM. Polyubiquitination of p53 by a ubiquitin ligase activity of p300. Science, 2003; 300: 342-344.
- Leprat F, Alapetite C, Rosselli F, Ridet A, Schlumberger M, Sarasin A, Suarez HG, Moustacchi E. Impaired DNA repair as assessed by the ‘comet’ assay in patients with thyroid tumors after a history of radiation therapy: a preliminary study. Int J Radiat Oncol Biol Phys, 1998; 40: 1019–1026.
- Prasad MA, Unqerback J, Ansberg J, Somasundaram R, Strid T, Laesson M, Paepe D, Lilljebjorn H, Haqman J, Sog-vardsson M. Ebf1 heterozygosity results in increased DNA damage in pro-B cells and their synergistic transformation by Pax5 haploinsufficiency. American society of hematol, 2014;125(26): 4052-9.
- Barquinero JF, Barrios L, Caballin MR, Miro R, Ribas M, Subias A, Egozcue J. Occupational exposure to radiation induces an adaptive response in human lymphocytes. Int J Radiat Biol,1995; 67 (2): 187-191.
- Betti C, Davini T, Giannessi L, Loprieno N, Barale R. Mi-crogel electrophoresis assay (comet test) and SCE analysis in human lymphocytes from 100 normal subjects. Mutat res, 1994; 307: 323-333.
- Fuchs RPP, Wanger J, Janel BR, Napolitano R. All three SOS-inducible DNA polymerases (Pol II, Pol IV and Pol V) are involved in induced mutagenesis. EMBO J, 2000;19 (22): 6259-6265.
| How to cite this article: Kaur S, Sangeeta, Galhna KK, Gautam N: Assessment of Radiation Induced DNA Damage in Human Peripheral Blood Lymphocytes Using COMET Assay. Int. J. Life. Sci. Scienti. Res., 2017; 3(4):1208-1214. DOI:10.21276/ijlssr.2017.3.4.17 Source of Financial Support: Nil, Conflict of interest: Nil |