ABSTRACT-
Plants of Polygalaceae family are source of several compounds such as xanthones, coumarins, phenols,
triterpenes, steroids, pyrones derivatives and alkaloids. These plants contain chemical compounds with a large spectrum of
biological activities, including anti-depressant and anti-angiogenic. Moutabea guianensis is an Amazonian species
belongs to the Polygalaceae family. In this work, from roots of M. guianensis were isolated a new xanthone,
3,8-dihydroxy-1,2,4,5-tetramethoxyxanthone, named moutabeone D, and one known xanthone, 1,3,5-trihydroxy-2-
methoxyxanthone. Column chromatography on silica gel and semi-preparative HPLC led the isolation of these
compounds. The structures were elucidated by spectroscopic data (HRESIMS, UV, IR, 1D and 2D NMR).
Key-Words- Moutabea guianensis, Polygalaceae, Xanthones
INTRODUCTION-
Moutabea Aublet is a Neotropical genus with
approximately 12 recognized species, including Moutabea
floribunda recently discovery [1]. Several authors already
mentioned the need of a taxonomic up-date and revision of
this genus [2]. The genus Moutabea belongs to the
Polygalaceae family which includes about 22 genera and
1300 species [3]. Several phytochemical investigations have
revealed that the family is a rich source of xanthones [4].
Although the chemistry of this family has been widely
studied [5-10], only one species, Moutabea guianensis has
been investigated among the Moutabea genus. Studies with
the roots of M. guianensis led to the identification of
xanthones [11-12]. Ongoing the chemical study on this plant
has now resulted in the isolation and identification of one
new xanthone identified as 3,8-dihydroxy-1,2,4,
5-tetramethoxyxanthone (1), named moutabeone D and the known xanthone 1,3,5-trihydroxy-2-methoxyxanthone (2)
(Fig. 1). In this paper, we describe the isolation and
structure elucidation of compound 1 and 2.
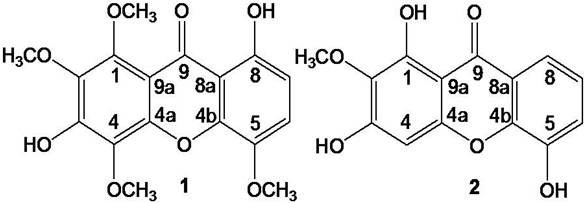
Fig 1: Structures of compounds 1 and 2 isolated from Moutabea guianensis
This work was carried out at the Laboratory of Chemistry of Natural Products at Federal University of Pará, in Belém City, State of Pará, Brazil, from March to October 2016.
Chemical reagents and Equipments- UV spectrum was obtained from LC equipped with DAD ProStar 335 (Varian, Palo Alto, CA, USA). IR was carried out on a Shimadzu Prestige 21 (Tokyo, Japan). NMR spectra were recorded on a Varian Mercury-300 NMR Spectrometer, operating at 300 MHz at 1H and 75 MHz at 13C, using d-chloroform as solvent. HRESIMS was carried out on a Waters Xevo G2-S QTof/Tof spectrometer (Milford, MA, USA). Column chromatography was performed on silica gel 60 (70-230 mesh, Macherey-Nagel, Düren, Germany). Precoated sheets of silica gel with UV254 indicator (thickness 200 µm) were used for TLC (Sorbent technologies, Norcross, GA, USA). Spots were visualized either with a UV lamp (254 nm) or by spraying with aqueous H2SO4 (50%) satured with CeSO4 solution, followed by heating. Semipreparative HPLC was carried out using a Varian Polaris with UV detector model ProStar 335 using a Phenomenex Gemini (Torrence, CA, USA) C18 column (250 mm x 10 mm, 5µm).
Plant material- Roots and stem of M. guianensis were collected on March 2012, in the experimental field of Embrapa Amazônia Oriental in the city Belém, state of Pará, Brazil. A voucher specimen (No. 195862) was deposited in the Herbarium MG of Museu Paraense Emílio Goeldi (Belém-PA, Brazil).
Extraction and isolation- Dry roots of Moutabea guianensis (928 g) were ground and successively percolated with hexane (3 L), ethyl acetate (3 L), methanol (3 L) at room temperature for five days each solvent. Solutions were evaporated to dryness under vacuum giving hexane, ethyl acetate and methanol extracts. The ethyl acetate extract (2.0 g) was submitted to CC using silica gel and mixtures of hexane, EtOAc and MeOH as eluents leding to 20 fractions of 125 mL each. The fractions were combined according to TLC in seven groups (G1-G7). Group G6 (982.3 mg) was purified by semi-prerative HPLC using an isocratic system of CH3CN-H2O 62:38, with a flow rate of 4.7 mL/min yielding compound 1 (8.1mg. tR 57.77 min). The stem of M. guianensis (2200 g) was subjected to the same process of roots. The ethyl acetate extract (4.9 g) was submitted to CC using mixtures of hexane, EtOAc and MeOH as eluents leding to 26 fractions of 125 mL each. The fractions were combined in eight groups (A1-A8). Group A5 (298.0 mg) was submitted to CC using mixtures of hexane and EtOAc as eluents in increasing order of polarity, collecting 123 fractions of 12 mL. The compound 2 (6.0 mg) was obtained from fraction 87, eluted with hexane/EtOAc (3:7).
RESULTS AND DISCUSSION- These chemical investigations led to the identification of two compounds. Compound 2 identified as 1,3,5-trihydroxy-2-methoxyxanthone had already been isolated from others species[13,14] and the compound 1 is reported here as new compound. Compound 1 (Figure 1) was obtained as yellow solid. Its molecular formula was determined to be C17H17O8 + by HRESIMS exhibiting the quasimolecular ion at m/z 349.0907 [M+H]+ (calcd. for C17H17O8 +, 349.0923), which indicated ten degrees of unsaturation. The 1H NMR showed seven signals, including four singlets attributed to the methoxy hydrogens (dH 3.95, 3.98, 4.02 and 4.13); two doublets ortho-coupled due the aromatic hydrogens at dH 6.70 (J = 9.0 Hz) and 7.21 (J = 9.0 Hz) and one singlet of a bonded hydroxyl group at dH 12.46. The 13C NMR and DEPT spectra showed sixteen signals, including a conjugated ketone carbonyl signal at dC 181.4 characteristic for a monochelated carbonyl carbon[15], twelve aromatic carbons signals, eight of them oxygen-substituted (dC 131.5 -154.9) and four oxygen- nonsubstituted (dC 108.7-120.2). In the same spectrum three methoxyl signals (dC 57.6, 62.1 and 61.7) were observed and the most intense of them (dC 61.7) was attributed to two methoxyl groups. The structure of compound 1 was also further deduced from HMBC spectrum. This spectrum shows that the ortho-coupled aromatic hydrogen at dH 7.21 (H-6) was correlated to the carbons of dC 154.9 (C-8), 139.7 (C-5) and 144.9 (C-4b). In addition, correlation of the methoxyl group (dH 3.95) with dC 139.5 (C-5) suggested the methoxyl group is linked to C-5. These correlations revealed that the ring A of 1 is similar to ring A of Angustin A [16]. The spectroscopic data above suggested that ring B has three methoxyl groups e one hydroxyl group as substituents. To confirm the methoxyl groups positions a NOE-diff experiment was carried out, which revealed spacial interactions between dH 3.98 (1-OCH3) and dH 4.13 (2-OCH3). Irradiation at dH 4.02 didn’t enhance the signals dH 3.98 and dH 4.13, which confirm that one methoxyl group is at C-4. NMR data are shown in Table 1. On the basis of these results, the structure of moutabeone D (1), was determined as 3,8-dihydroxy-1,2,4,5- tetramethoxyxanthone.
Table 1: 1H-NMR (300 MHz) and 13C-NMR (75 MHz) spectral data for compound 1 in CDCl3
| Positions | δH (J in Hz) | δC | DEPT | HMBC (H→C) |
|---|---|---|---|---|
| 1 | - | 148.9 | C | |
| 2 | - | 131.5 | C | |
| 3 | - | 148.4a | C | |
| 4 | - | 137.9 | C | |
| 4a | - | 147.1a | C | |
| 4b | - | 144.9 | C | |
| 5 | - | 139.7 | C | |
| 6 | 7.21 (d, 9.0) | 120.2 | CH | 4b, 5, 8 |
| 7 | 6.70 (d, 9.0) | 109.0 | CH | 5, 8, 8a, 9 |
| 8 | - | 154.9 | C | |
| 8a | - | 109.2 | C | |
| 8b | - | 108.7 | C | |
| 9 | - | 181.4 | C | |
| 1-OCH3 | 3.98 (s) | 62.1 | CH3 | 1 |
| 2-OCH3 | 4.13 (s) | 61.7 | CH3 | 2 |
| 4-OCH3 | 4.02 (s) | 61.7 | CH3 | 4 |
| 5-OCH3 | 3.95 (s) | 57.6 | CH3 | 5 |
| 3-OH | No observed | - | - | |
| 8-OH | 12.46 (s) | - | - |
Spectroscopy data-
Compound 1.- UV ?max /nm (acetonitrile-water): 204, 233, 278, 306, 373 (sh). IR (KBr) 3757, 3273, 2935, 2844, 2350, 1957, 1591, 1477, 1350, 1237, 1056, 966, 890, 804, 727 cm-1. 1H –NMR (300 MHz, CDCl3) and 13C-NMR (75 MHz, CDCl3) data, see Table 1. HRESIMS: m/z 349.0907 ([M+H]+, C17H17O8 +; calcd. 349.0923).
Compound 2.- 1H –NMR (300 MHz, CDCl3): dH 6.54 (s, H-1), 7.35 (dd, J=7.5 e 1.8 Hz, H-6), 7.28 (dd, J=8.0 e 7.5 Hz, H-7), 7.67 (dd, J=8.0 e 1.8 Hz, H-8), 3.88 (s, 2-OCH3); 13C-NMR (75 MHz, CDCl3): dc 155.5 (C-1), 131.7 (C-2), 153.9 (C-3), 94.8 (C-4), 159.3 (C-4a), 146.2 (C-4b), 147.0 (C-5), 121.4 (C-6), 124.9 (C-7), 116.3 (C-8), 121.9 (C-8a), 182.3 (C-9), 104.1 (C-9a), 60.9 (2-OCH3).
CONCLUSION- Moutabea guianensis belonging to the Polygalaceae family is a species with few chemical studies. The chemical study of roots and stem from M. guianensis led to isolation of two more xanthones for this specie. The compounds were isolated using High Performance Liquid Chromatography (HPLC) and Column Chromatography (CC), and structural characterization of compounds was established on the basis of spectroscopic methods, mainly 1D and 2D nuclear magnetic resonance (NMR). One of them 3,8-dihydroxy- 1,2,4,5-tetramethoxyxanthone, named moutabeone D, it is a new natural product. The other compound, 1,3,5-trihydroxy-2-methoxyxanthone, was isolated for the first time in this genus. The results presentated in this paper show that Moutabea genus is a great natural source of xanthones and needs further studies.
ACKNOWLEDGEMENT- The authors are grateful to the National Counsel of Technological and Scientific Development (CNPq), Coordination for the Improvement of Higher Education Personnel (CAPES), Pro-Rectory of Research and Post-graduation at UFPA (PROPESP) and Amazon Foundation for Support to Studies and Research of Pará (FAPESPA) for financial support.
REFERENCES
- Silveira JB, and Secco RS. A new species of Moutabea (Polygalaceae) for the Brazilian Amazon, Guyana and Peru. Phytotaxa, 2015; 202(4): 259-265.
- Jansen-Jacobs MJ, and Maas PJM. Moutabea arianae, a new species of Polygalaceae from French Guiana and adjacent Brazil. Blumea, 2010; 55(1): 86-87.
- Mesquita AS, Rocha AS, and Santos JU. Polygalaceae nas restingas do estado do Pará, Brasil. Rev Bras Biociênc, 2013; 11(1): 76-87.
- Negi JS, Bisht VK, Singh P, Rawat MSM, and Joshi GP. Naturally occurring xanthones: Chemistry and biology. Appl Chem, 2013; 1-9.
- Klein Junior LC, Gandolfi RB, Santin JR, Lemos M, Cechinel Filho V, and Andrade SF. Antiulcerogenic activity of extract, fractions, and some compounds obtained from Polygala cyparissias St. Hillaire e Mouquin (Polygalaceae). N.-S. Arch Pharmacol, 2010; 381(2): 121-126.
- Yang X, Xu L, and Yang S. Xanthones from the stems of Securidaca inappendiculata. Phytochemistry, 2001; 58(8): 1245-1249.
- Bergeron C, Marston A, Wolfender JL, Mavi S, Rogers C, and Hostettmann K. Isolation of polyphenols from Polygala gazensis and liquid chromatography-mass spectrometry of related African Polygala species. Phytochem Anal, 1997; 8(1): 32-36.
- Pinheiro TR, Cechinel V, Santos ARS, Calixto JB, Delle-Monache F, Pizzolatti MG, and Yunes RA. Three xanthones from Polygala cyparissias. Phytochemistry, 1998; 48(4): 725-728.
- Pizzolatti M, Cunha A, Pereira WS, and Delle Monache FA. A new styryl-2-pyrone derivative from Polygala sabulosa (Polygalaceae). Biochem Syst Ecol, 2004; 32(7): 603-606.
- Rocha JLC, Pastore JFB, Brandão HN, Azevedo A, Devid JP, Santos EO, and David JM. Quantificação de salicilato de metila em quatro gêneros de Polygalaceae, por CLAE-DAD. Quim Nova, 2012; 35(11): 2263-2266.
- Ripardo Filho HS, Pacheco LCS, Andrade ES, Correa MJC, Guilhon, GMSP, and Santos LS. A new xanthone from Moutabea guianensis Aubl. Molecules, 2014; 19(7): 8885-8889.
- Ripardo Filho HS, Pacheco LC, Andrade ES, Correa MJC, Araújo RNM, Guilhon GMSP, and Santos LS. Xanthones from the roots of Moutabea guianensis Aubl. Molecules, 2015; 20(1): 127-134.
- Mesquita AAL, Oliveira WG, Neiva RMT, and Gottlieb OR. Xanthones from Tovomita pyrifolium. Phytochemistry, 1975; 14(3): 803-806.
- Morel C, Séraphin D, Oger J, Litaudon M, Sévenet, T, and Bruneton J. New xanthones from Calophyllum caledonicum. J Nat Prod, 2000; 63(11): 1471-1474.
- Van der Sluis WG, and Labadie RP. Polyoxygenated xanthones of Centaurium littorale. Phytochemistry, 1985; 24(11): 2601-2605.
- Zhu B, Zhe W, Duan Y, Wang M, Gao Y, Wei G, and Liao T. Two new xanthones from Swertia angustifolia. J Asian Nat Prod Res, 2012; 14(2): 154-158.
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |
| How to cite this article: Ripardo Filho HS, Pacheco LC, Da Silva EA, Guilhon GMSP, Santos LS: A New Hexaoxygenated Xanthone from the Roots of Moutabea guianensis Aubl. Int. J. Life. Sci. Scienti. Res., 2017; 3(1): 817-819. DOI:10.21276/ijlssr.2017.3.1.11 Source of Financial Support: CNPq- FAPESPA, Conflict of interest: Nil |