ABSTRACT-
The adult plant resistance (APR) gene Lr34 and Lr46 of wheat is associated with leaf tip necrosis (Ltn),
and provide resistance against multiple diseases, viz. leaf rust, stripe rust, stem rust, powdery mildew and spot blotch.
APR gene Lr46 is also associated with Ltn. Lesion mimic (lm) mutants express hypersensitive responses in the absence of
pathogens and also confer resistance to biotrophic pathogens, including leaf rust. However, association between spot
blotch and Ltn is reported, but not with Lm and Ltn. Five hundred diverse lines of spring wheat including two hundred
and ninety four wami population including were screened for Lr34 (CsLv34, 150 bp), Lr46 (STS1BL2, 600bp) and three
lesion mimic genes, viz. lm (Xwmc85.1 and Xgwm264) on 1B, lm1 (Xbarc147 and Xwmc674) on 3BS, and lm2 (Xgwm513
and Xgwm149) on 4BL.Lr34alone was found in only one line, but in 31% of lines it was in combination with Lr46. In
contrast, Lr46 was present singly in 63% of lines. The Lm genes appeared to increase spot blotch severity whereas Lr34
alone or with Lr46 provided moderate to high level of resistance. Lm expression was absent when both Lr34 and Lr46
were present indicating their masking effect on Lm genes. The presence of Lr46 alone showed variable Lm expression.
This suggests that the presence of Lr34 + Lr46 and/or absence of Lm genes enhance resistance to spot blotch.
Key-Words- Leaf tip necrosis (Ltn), Lesion mimics (Lm), Spot blotch, Phenotypic marker, Adult plant resistance (APR)
Lr34 confers horizontal resistance or slow rusting resistance although under appropriate conditions it may confer resistance in seedlings to certain rust races. The efficianacy of Lr34 mediated APR also varies between different cultivar backgrounds and growth conditions. More importantly Lr34 has been recognized as major component of durable rust resistance as it can act synergistically with other leaf rust resistance genes. Lr34 encodes a putative adenosine triphosphate–binding cassette (ABC) transporter. It belongs to the ABC subtype G (formerly PDR), subclass of transporters [5]. It was reported that developmentally regulated resistance is correlated with elevated Lr34 transcript levels in flag leaves of adult plants. There are several allelic forms of the Lr34 gene that have been identified in the wheat germplasm, with two alleles occurring at a high frequency [6]. Resistance allele (Lr34res) differs by only two amino acid changes from the other allelic forms associated with susceptibility (Lr34sus) and so gain-of its function of resistance. So it is assumed that the resistance conferred by Lr34res is the result of altered activity or substrate specificity. The presence of Lr34 and Lr46 in wheat is associated with leaf tip necrosis (Ltn), a type of necrosis that forms predominantly in the tips of wheat flag leaves, and is dependent on expression of Lr34res as well as on genetic. The gene Lr34 was postulated in few Indian bread wheat cultivars reported the association of leaf tip necrosis with the spot blotch of wheat [7].
Spot blotch caused by Bipolaris sorokiniana has emerged an important problem in the last century in 9 million hectare what of South East Asia. This pathogen affects the crops of millions of small and marginal farmers of this region and cause severe damage. Annual average yield losses due to spot blotch are reported around 19.6 and 15.5 % in wheat [8]. There are many program have been initiated to improve the wheat against spot blotch [9-10]. But not much success can be achieved to reduce or minimize the damage due to spot blotch. Due to complex problems of pathogen and tropical environment of South Asia Breeding progress is slow. Substantial gain to spot blotch resistance as made by sleeting wheat lines with Ltn [7]. [11] reported the association of Lr34 and Lr46 with the spot blotch resistance. In present studied population genotyping with SSR markers shows the predominance of Lr46 alone or in combination of Lr34. This study was performed for variable level of spot blotch severity to understand the role of different gene combination in the spot blotch resistance. Spot blotch spreading is very much influenced by environmental condition like temperature and radiation. It was reported that an increase in these two factors also shows additive effect in the susceptibility. Terminal heat stress increased severity and showed positive correlation in South Asia [10,12-13]. Temperature is an important non living factor that influenced the developmental growth stages of plant. The total temperature required for plant growth development can be calculated by accumulating degree days between the high and low temperature thresholds throughout the crop season [14]. Incidence and spread of spot blotch are influenced by the growth stages, and maturity duration of wheat [15]. Plants that spontaneously express necrotic lesions during development or in response to changes in the environment, without the presence of any pathogen, are denoted as lesion mimics exhibiting either constitutive or unregulated cell death like HR after pathogen infection. So it can be hypothesized that Lm in wheat facilitating the spot blotch development and its rapid spread in the host due to readily available necrotic tissue for colonization of the pathogen. If this is true then Lm free/least Lm wheat lines expected to have low susceptibility and Lm expression should also be suppressed by Ltn like spot blotch so the objective of this study was to establish association between Ltn that is a trait of resistance, lm and spot blotch, phenotypic and genotypic expression and relation of different Ltn genes and distribution of different Ltn genes in present population.
MATERIALS AND METHODS-
Screening of Germplasm: Five hundred diverse wheat germplasm including two hundred ninety four wami germplasm were evaluated for leaf rust caused by Puccinia recondite, spot blotch causal organism Bipolaris sorokiniana and Lesion mimic at the Research Farm of Banaras Hindu University, Varanasi during crop season 2013-14, 2014-2015 and 2015-16.
Sowing and maintenance of crop under field and polyhouse condition: Banaras Hindu University falls in the rice wheat cropping system zone. The field was prepared by ploughed thrice after the harvest of rice crop. Fertilizers NPK were applied @ 60, 60 and 40 Kg/ha during land preparation. Five hundred wheat germplasm were sown in the alpha lattice design in two replication, 25 blocks and each block carrying 20 genotypes. Seeds were sown manually keeping row to row distance 25 cm and plant to plant 12 cm. The first irrigation was given at crown root initiation 20 days after sowing and 30 Kg Nitrogen was also given as top dressing after 3 days of irrigation while the last dose of nitrogen was given after flowering (GS45).
Scoring of leaf tip necrosis: Leaf tip necrosis was scored on flag leaf in the field at the GS 47 to 73. Flag leaves showing necrosis from the tip and extended to 5cm were counted as Ltn. Leaf tip necrosis was not appeared in poly house therefore it was not scored. Under poly house conditions genotypes were screened at GS 47-73 before ear emergence. Leaves were scored in two way, 1st it was scored as presence or absence of Ltn. 2nd when it was present it was scored as the level of their expression. The scale was used for it is 0 to 4 where 0= no Ltn, 1=25%, 2=50%, 3=75% and 4 more than 75%.
Scoring of lesion mimic: The plants were observed from seedling to adult stage both in field and polyhouse for the expression of Lm symptoms. Lesion mimic was recorded from seedling (GS25) to adult plant stage (GS60) in poly house. Lm phenotype was scored on flag leaf in the field and poly house at the GS 60. The distribution of Lm on leaf i.e. apical, middle and basal part was also recorded. Leaves showing typical LM expression was scored with modification of 1- 6 rating scale of [16] in 1-9 scale. Where 0=no visible specks 1= 1-10%, 2=10.1 to 20%, 3= 20.1-30,4= 30.1-40, 5= 401-50, 6= 50.1-60, 7= 60.1-70, 8, 70.1-80, 9= above80%.
Isolation of pathogen: All the genotypes produced leaf tip necrosis were collected for the presence of Bipolaris sorokiniana or other pathogen. Three well development lesions were taken for the isolation of pathogen. A piece of 1mm2 leaf tissue was taken out from the one week old lesion. Isolation was performed by the method of [17].
Inoculation of the pathogen: A pure culture of the most aggressive isolate of Bipolaris sorokiniana (NABM MAT 1; NCBIJN128877, Banaras Hindu University, Varanasi, India) known to be highly aggressive was used to create artificial epiphytotic [7]. The isolate was multiplied on sorghum grain and a spore suspension adjusted to 10-4 spores/ml of water using haemocytometer was uniformly sprayed at GS 50 on Zadoks scale during evening hours [18].
Disease assessment: Spot blotch score for each line was evaluated on five randomly tagged plants in the field at three different growth stages (GS) viz; GS 63 (beginning of anthesis to half complete), GS 69 (anthesis complete) and GS 77 (late milking) [18] following double digit scale (DD, 00-99) [19]. The first digit (D1) indicates vertical disease progress on the plant and the second (D2) indicates severity measured in diseased leaf area. For each score, the disease severity percentage was based on the following formula: % severity = (D1/9) (D2/9)100.
Area under the disease progress curve based on disease severity over time at GS63, GS69 and GS77 was calculated using the percent severity estimations corresponding to the disease ratings, as outlined by:
Where, Yi= disease level at time ti
t(i + 1) - ti = Time (days) between two disease scores
n = number of dates at which spot blotch was recorded
Use of molecular markers for the leaf rust resistance genes-
Molecular marker analysis- A total of 2 SSR and STS markers associated with Ltn and 10 SSR markers associated with lesion mimic mutants, were used for screening of wheat germplasm viz; CsLv34(7DS) and STS1BL2 (1BL), Xwmc 674 (3BS); Xbarc1033 (3BS); Xbarc147 (3BS); Xgwm513 (4BL); Xgwm149 (4BL); Yao et al., 2009 Xbarc181 (1B); Xbarc61 (1B); Xgwm131 (1B); Xwmc85.1 (1B); Xgwm264.1 (1B) [20].
DNA extraction, PCR amplification and gel electrophoresis- Plant gnomic DNA extracted from the leaves of 15 days old seedling by following the modified CTAB method [21]. Isolated DNA was kept at -20°C for further use. Each Polymerase chain reaction (PCR) was performed in 15µl reaction volume containing 10ng genomic DNA, 1.5µl 10nM Tris-HCL (pH 8.8), 0.2µl dNTPs (MBI Fermentas), 0.2µl 2.5mM MgCl2 (MBI fermentas), 0.2µl Taq polymerase 5U/µl (MBI fermentas) and 0.6µl forward and reverse primers. The sequences of each primer are listed in Table 1. Amplification were performed in touchdown i.e., 10 cycles were performed at 94°C for 1min, 65-55°C for 1min and 72°C for 1min. After completion of one cycle, the annealing temperature lowers down 1°C. Remaining 30 cycles were performed at 94°C for 30s, 55°C for 30s and 72°C for 30s with a final extension at 72°C for 7 min. The 15µl PCR products were mixed with 2µl loading dye (98% formamide, 0.3% of each bromophenol blue and xylene cyanol, and 10mM of EDTA). Electrophoresis was carried out in 2.5% agarose gel stained with EtBr and 1 x TAE gel electrophoresis buffer, prepared from 50 x TAE (242g Tris-base, 57.1ml Glacial acetic acid and 100ml 0.5M EDTA for 1L), was used for electrophoresis, at 75 W for 2 hours. Gels picture was visualized under UV light by using UVP gel doc.
RESULTS-
Experiment in Field and Poly house conditions- Ltn were first observed on the leaves of plants. The expression of Ltn in all plant of same genotypes was uniform however it was un-uniform in few genotypes. The appearance of Ltn was recorded at late GS (47 to 73). Lm symptoms were first observed on the leaf at the time of booting stage and scored.The genotypes with good expression of Ltn show no or very less expression of lm (Fig. 1). Appearance and distribution of Ltn under poly-house was not like the field. Ltn in poly house was not expressed. There were no plant in poly house expressed Ltn.
Effect of Temperature on expression of Ltn- During the experiment it was observed that no expression of Ltn was seen in poly house (DD 1107) however it was clearly expressed in field (DD 840). So it can be predict that Ltn and DD are negatively associated. At the time of experiment maximum temperature in field was 280C and in polyhouse it was above 400C. So it can also be predicted that at high temperature the Ltn did not expressed itself. So it is clear that gene of Ltn is thermolabile. Appearance and distribution of Lm under poly-house was different than the field. Under poly-house more than 70% genotypes expressed Lm at early (GS 25-30) (35 days after showing). The Lm severity was higher in polyhouse than the field it may be due to absence of Ltn and presence of high temperature.
Leaf tip necrosis and its association with spot blotch- In the field Ltn and spot blotch were recorded in some of different way as previously reported. All those lines which express Ltn very well were not resistant against spot blotch. Some lines with Ltn were also susceptible for the disease, but some of the Ltn lines shows less severity or no severity of disease. The susceptible genotypes which have Ltn in their phenotype show the higher AUDPC (Table 2).
Genotyping for Ltn lines- Five hundred diverse lines including two hundred ninety four wami population of spring wheat were screened for Lr34 (CsLv34), Lr46 (STS1BL2). Genotyping of Ltn lines clearly showed the presence of two different genes i.e.Lr34, and Lr46 (Fig. 2). The frequency of these genes alone and in combinations is given in table 2.In the populationLr34 alone was found in only four line, but in 31% of lines it was in combination with Lr46 (Table 1). In contrast, Lr46 was present singly in 63% of lines. Lr34 alone or with Lr46 provided moderate to high levels of resistance.
Leaf tip necrosis and its association with spot blotch- The genotyping shows that Ltn with Lr46 does not shows the resistance against disease but if the gene Lr34 is present then they were shown good resistance against the disease. Lr34 provides more resistance against spot blotch in comparison to Lr46 (Fig 1, Table 4).
Lm, Ltn and Spot Blotch in field- The lines with Ltn which have less or no expression of lesion mimic were resistance or moderately resistance against spot blotch. Table 1, shows the distribution of Ltn in wheat genotypes and Table 2 shows slow or no Lm frequency on those genotypes where Ltn was present.
Ltn, Lm and DD- Lm and DD (degree days) were positively correlated whereas Ltn and DD are negatively correlated. Lesion mimic expression was recorded after 35 days of sowing in the poly house when it received the total DD 1101.1. Whereas on the same genotypes LM appeared after 74 days under filed conditions with the DD 740 (Table 4.) but in case of Ltn in polyhouse at the 1101DD it does not appear and in field at 840 DD it was appeared very well (Fig 1, Table 3). Ltn is thermolabile where lm is thermostable.
Table 1. Distribution of LTN genes among 500 wheat genotypes
| Ltn gene (S) | Number of genotypes | % Germplasm | Ltn gene (S) |
|---|---|---|---|
| Lr34 | 4 | 0.8 | Lr34 |
| Lr46 | 315 | 63 | Lr46 |
| Lr34+Lr46 | 155 | 31 | Lr34+Lr46 |
Table 2. List of wheat genotypes without Lesion mimic expression in the field and poly house, status of Lm gene, Ltn and yield related characters. (a- absent, p- present)
| Entry | Genotype | DH | AUDPC | PLOT YIEL D | TKW | Lm field | lm Poly- House | lm | lm 1 | lm 2 | Ltn |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | VORONA/GEN | 87 | 417.22 | 119.5 | 31.8 | A | a | p | p | P | p |
| 2 | PARA2// JUP/BJY/3/VEE/JUN/4/2*K AUZ | 86 | 564.45 | 55 | 24.8 | A | a | p | a | P | p |
| 3 | KAUZ/RAYON | 82 | 447.66 | 125.5 | 25 | A | a | p | p | P | p |
| 4 | RABE/2*MO88 | 86 | 438.09 | 114.5 | 24.8 | A | a | p | p | P | p |
| 5 | OASIS/SKAUZ//4*BCN | 86.5 | 365.81 | 110 | 22.6 | A | a | p | a | P | p |
| 6 | HUAYTU CIAT | 83 | 406.73 | 116.5 | 30.2 | A | a | p | p | A | p |
| 7 | TARACHI F 2000 | 86.5 | 453.03 | 107.5 | 30.4 | A | a | p | a | P | p |
| 8 | TAURUM | 86.5 | 366.48 | 63 | 21.6 | A | a | p | p | P | p |
| 9 | CNDO/R143//ENTE/MEXI_2/3/A EGILOPSSQUARROSA (TAUS)/4/WEAVER/5/2*KAUZ | 84 | 351.92 | 148 | 30 | A | a | p | p | P | p |
| 10 | KETUPA*2/PASTOR | 86.5 | 573.21 | 104 | 20.3 | A | a | p | p | P | a |
| 11 | REH/HARE//2*BCN/3/CROC_1/ AE.SQUARROSA (213)//PGO/4/HUITES | 82 | 523.77 | 147 | 32.4 | A | a | p | p | P | a |
| 12 | CROC_1/AE.SQUARROSA (205)//BORL95/3/PASTOR | 84 | 490.81 | 107 | 29.8 | A | a | p | p | A | a |
| 13 | ATTILA/3*BCN*2//BAV92 | 86 | 396.05 | 130 | 25.4 | A | a | p | a | P | a |
| 14 | TUKURU//BAV92/RAYON | 82 | 562.22 | 128 | 28.3 | A | a | a | a | P | p |
| 15 | WBLL1*2/4/YACO/PBW65/3/KA UZ*2/TRAP//KAUZ | 84 | 461.24 | 134 | 28.4 | A | a | p | p | P | p |
| 16 | PAVON | 87 | 436.91 | 106.5 | 22.8 | A | a | p | p | P | p |
| 17 | KEA/BUC//FCT | 80 | 505.13 | 127.5 | 33.2 | A | a | p | p | P | p |
| 18 | PRL/SARA//TSI/VEE#5 | 84 | 557.8 | 132 | 30.2 | A | a | p | p | P | a |
| 19 | CNDO/R143//ENTE/MEXI_2/3/A EGILOPS SQUARROSA (TAUS)/4/WEAVER | 83.5 | 520.75 | 162 | 36.6 | A | a | a | p | P | p |
| 20 | CROC_1/AE.SQUARROSA (205)//JUP/BJY/3/SKAUZ/4/KAU Z | 86 | 409.88 | 151.5 | 30.6 | A | a | p | p | P | p |
| 21 | PASTOR// SITE/MO/3/CHEN/AEGILO PS SQUARROSA (TAUS)//BCN | 82 | 425.06 | 151.5 | 36.8 | A | a | a | a | A | p |
| 22 | BARBET1 | 84 | 502.9 | 124.5 | 27.2 | A | a | p | p | P | p |
| 23 | MILVUS2 | 82 | 540.06 | 130.5 | 29.2 | A | a | p | p | p | p |
| 24 | ATTILA*2/PBW65 | 82 | 439.33 | 127 | 27.8 | A | a | p | p | P | p |
| 25 | VOROBEY | 86.5 | 366.05 | 121.5 | 25.4 | A | a | p | p | P | p |
| 26 | MILAN/KAUZ//PRINIA/3/BAV92 | 84 | 443.83 | 115.5 | 30.6 | 0 | p | p | P | p | |
| 27 | PASTOR/DHARWAR DRY | 74 | 211.85 | 157.5 | 34.2 | 0 | 0 | a | a | A | p |
| 28 | MILAN/ KAUZ/3/URES/JUN//KAUZ /4/CROC_1/AE.SQUARROSA (224)//OPATA | 81.5 | 239.14 | 159 | 36.9 | A | a | p | p | P | p |
| 29 | CNO79//PF70354/MUS/3/PASTO R/4/CROC_1/AE.SQUARROSA (224)//OPATA | 84 | 522.66 | 118.5 | 28.4 | A | a | p | p | P | |
| 30 | CROC_1/AE.SQUARROSA (224)//OPATA/3/BJY/COC//PRL/B OW/4/BJY/COC//PRL/BOW | 82 | 454.32 | 110.5 | 29.4 | A | a | a | a | P | a |
| 31 | CHEN/AE.SQ//2*OPATA/3/BAV9 2/4/JARU | 82 | 434.26 | 132 | 32 | A | a | a | a | P | p |
| 32 | CHONTE | 86.5 | 528.53 | 141.5 | 31.2 | A | a | p | a | A | p |
| 33 | WHEAR//2*PRL/2*PASTOR | 80 | 548.34 | 110 | 31.6 | A | a | a | p | P | p |
| 34 | KAUZ//ALTAR 84/AOS/3/MILAN/KAUZ/4/HUIT ES | 82 | 536.55 | 144 | 32 | A | a | p | p | P | p |
| 35 | CROC_1/AE.SQUARROSA (205)//BORL95/3/PRL/SARA//TSI /VEE#5/4/FRET2 | 84 | 498.15 | 103.5 | 24.4 | A | a | p | a | a | p |
| 36 | CROC_1/AE.SQUARROSA (205)//BORL95/3/PRL/SARA//TSI /VEE#5/4/FRET2 | 84 | 538.83 | 101.5 | 26.2 | A | a | p | p | P | p |
| 37 | ALTAR 84/AE.SQUARROSA (219)//OPATA/3/WBLL1/FRET2// PASTOR | 81.5 | 345.74 | 135 | 34 | A | a | p | p | A | p |
| 38 | YAV_3/SCO//JO69/CRA/3/YAV79 /4/AE.SQUARROSA (498)/5/2*OPATA | 82 | 177.66 | 159 | 31 | A | a | p | p | P | p |
| 39 | YAV_3/SCO//JO69/CRA/3/YAV79 /4/AE.SQUARROSA (498)/5/2*OPATA | 83.5 | 487.04 | 104 | 27.8 | A | a | p | p | P | p |
| 40 | CHEWINK | 84 | 214.75 | 158.5 | 28.4 | A | a | a | p | P | p |
| 41 | THB/KEA//PF85487/3/DUCULA/ 4/WBLL1*2/TUKURU | 82 | 585.43 | 147 | 33.4 | A | a | a | a | A | p |
| 42 | WBLL1*2/3/SNI/TRAP#1//KAUZ *3/TRAP/4/KACHU | 85.5 | 395.99 | 133.5 | 33.6 | A | a | a | a | A | a |
| 43 | PBW343*2/KUKUNA*2//WHEA R | 81.5 | 375.74 | 140 | 32.8 | A | a | p | a | P | p |
| 44 | MILAN/ KAUZ//PRINIA/3/BAV92/5/ TRAP#1/BOW//VEE#5/SARA/3/Z HE JIANG 4/4/DUCULA | 82 | 373.77 | 119 | 31 | a | a | p | p | P | p |
| 45 | ASTREB/OAX93.10.1//SOKOLL | 82 | 211.14 | 131 | 31.6 | A | a | p | p | P | p |
| 46 | BACANORA T 88 | 80 | 534.14 | 105.5 | 24.2 | A | a | p | a | P | p |
| 47 | BECARD/KACHU | 84 | 485.99 | 119.5 | 29 | A | a | p | p | P | p |
| 48 | C80.1/3*BATAVIA//2*WBLL1/5/ REH/HARE//2*BCN/3/CROC_1/ AE.SQUARROSA (213)//PGO/4/HUITES | 85.5 | 506.18 | 133.5 | 27.2 | A | a | p | p | P | p |
| 49 | KFA/3/PFAU/WEAVER//BRAMB LING/4/PFAU/WEAVER*2//BRA MBLING | 87 | 372.16 | 138 | 29.7 | A | a | p | p | P | p |
| 50 | MILAN/KAUZ//PRINIA/3/BAV92 | 82 | 439.39 | 128 | 30.5 | A | a | p | p | P | a |
| 51 | QUAIU #3//MILAN/AMSEL | 86.5 | 472.22 | 143.5 | 28.2 | A | a | p | p | A | p |
| 52 | TACUPETO F2001/SAUAL/4/BABAX/LR42// BABAX*2/3/KURUKU | 86.5 | 474.45 | 125 | 28.6 | A | a | p | p | P | a |
| 53 | TRCH//INQALAB 91*2/KUKUNA | 76 | 430.87 | 92.5 | 28.4 | A | a | a | a | P | p |
| 54 | CROC_1/AE.SQUARROSA (205)//KAUZ/3/SASIA/4/TROST | 82 | 420.07 | 135 | 31.4 | A | a | p | p | P | p |
| 55 | ESDA//ALTAR 84/AE.SQUARROSA (211)/3/ESDA/4/CHOIX/5/WAXW ING | 82 | 503.42 | 137 | 28.6 | A | a | p | p | P | a |
| 84 | 417.78 | 145 | 34.8 | A | a | a | a | P | p |
Table 3. Cumulative degree days in poly house and field
| Field | Poly House | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Week No. | Month and Date | Min-Max | Da ys | Max | Min | Digreedays | Min- Max | Days | Max | Min | Digree days |
| 50 | Dec 10-16 | 13.6-26 | 2 | 0 | 0 | 18.3 | 0 | 0 | 0 | 0 | 0 |
| 51 | Dec 17-23 | 6.7-26 | 7 | 169.1 | 73.5 | 51.3 | 0 | 0 | 0 | 0 | 0 |
| 52 | Dec 24-31 | 5-22.2 | 8 | 145.4 | 58.8 | 22.1 | 1 | 28 | 40 | 24 | |
| 1 | Jan1-7 | 2.5-25 | 7 | 138.8 | 50 | 24.4 | 192 | 291 | 171.5 | ||
| 2 | Jan 8-14 | 2.8-27 | 7 | 142.7 | 39.9 | 21.3 | 26-46 | 194 | 296 | 175 | |
| 3 | Jan 15-21 | 6.5-32.5 | 7 | 183.3 | 82.4 | 62.85 | 26-42 | 192 | 288 | 170 | |
| 4 | Jan 22-28 | 4-22.5 | 7 | 144.8 | 41.3 | 23.05 | 31-43 | 227 | 296 | 191.5 | |
| 5 | Jan 29-Feb4 | 6.6-26 | 7 | 171 | 63.7 | 47.35 | 31-50 | 249 | 312 | 210.5 | |
| 6 | Feb 05-11 | 8-27.7 | 7 | 177.6 | 79.5 | 58.55 | 36-48 | 186 | 232 | 159 | |
| 7 | Feb 12-18 | 11.6-28 | 7 | 179.1 | 93.8 | 66.45 | 0 | 0 | 0 | ||
| 8 | Feb 19-25 | 11.8-28 | 7 | 176.2 | 92.3 | 64.25 | 0 | 0 | 0 | ||
| 9 | Feb 26- Mar04 | 13.1-30 | 7 | 199.6 | 98.8 | 79.2 | 0 | 0 | 0 | ||
| 10 | Mar 5-11 | 13.5-35 | 7 | 219.1 | 101.7 | 90.4 | 0 | 0 | 0 | ||
| 11 | Mar 12-18 | 16.4-33.8 | 7 | 232.5 | 128.6 | 110.55 | 0 | 0 | 0 | ||
| 740.05 | 1101.5 | ||||||||||
Table 4. Correlation coefficient of Lesion mimics with Ltn and other yield related traits
| Character | 2013-14 | 2014-15 | 2015-16 |
|---|---|---|---|
| Ltn | -0.87 | -0.99 | -0.85 |
| DH | -0.070* | -0.187** | -0.099** |
| AUDPC | 0.088** | 0.141** | 0.193** |
| PlotYield | -0.102** | -0.116** | -0.003 |
| TKW | -0.085** | -0.075* | -0.050 |

Fig 1. Association of lesion mimics with Ltn and spot blotch
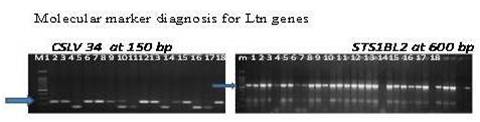
Fig 2. Screening of Ltn genes in present studied population

Fig 3. Different pattern of expression of Ltn on wheat plant in the field
The increase in global heat or temperature also cause the threatened of escape of Ltn gene expression due to its thermolabile nature.
CONCLUSION- Present investigation confirms that alone Lr34 or Lr46 does not provide desire level of resistance but both the genes in conjugation provide a good level of resistance against spot- blotch and mask the expression of lesion mimic. So in South Asia region the variety having both the genes is more useful for food safety and yield loss. Ltn inhibit the expression of lesion mimic phenotypically.
ACKNOWLEDGMENT- The authors are thankful for the financial support to UGC and CGIAR and CYMMIT for research material.
REFERENCES
- Dyck PL. The association of a gene for leaf rust resistance with the chromosome-7D suppressor of stem rust resistance in common wheat. Genome, 29, 467–469.and sturdy wheats. Crop Sci, 1982; 31, 309–311.
- Singh RP and Rajaram S. Resistance to Puccinia recondita sp. Tritici in 50 Mexican bread wheat cultivars. Crop Sci, 1991; 31: 1472-1479.
- Singh RP. Expression of wheat leaf rust resistance gene Lr34 in seedlings and adult plants. Pl. Dis, 1992; 76: 489-491.
- Das S, Aggarwal R, and Singh DV. Differential induction of defense related enzymes involved in lignin biosynthesis in wheat in response to spot blotch infection. Indian Phytopathology, 2003; 56, 129–133.
- Krattinger SG, Lagudah ES, Spielmeyer W, Singh RP, Huerta-Espino J, McFadden H, Bossolini E, Selter LL and Keller B. A putative ABC transporter confers durable resistance to multiple fungal pathogens in wheat. Science, 2009; 323, 1360–1363.
- Dakouri A, McCallum BD, Walichnowski AZ and Cloutier S. Finemapping of the leaf rust Lr34 locus in Triticum aestivum (L.) and characterization of large germplasm collections support the ABC transporter as essential for gene function. Theor. Appl. Genet, 2010; 121, 373–384.
- Joshi AK, Chand R, Kumar S and Singh RP. Association of leaf tip necrosis with the spot blotch pathogen in wheat. Crop Sciences, 2004; 44:792- 797.
- Dubin HJ and Van Ginkel M. The status of the wheat diseases and disease research in warmer areas. In D. A. Saunders (Ed.), Wheat for the Nontraditional warmer Areas. Mexico, DF: CIMMYT, 1991; pp. 125–145.
- Joshi AK, Kumari M, Singh VP, Reddy CM, Kumar S, Rane J and Chand R. Stay green trait: variation, inheritance and its association with spot blotch resistance in spring wheat (Triticumaestivum L.). Euphytica, 2007b; 153, 59–71.
- Sharma RC, Duveiller E. Effect of Helminthosporium leaf blight on performance of timely and late-seeded wheat under optimal and stressed levels of soil fertility and moisture. Field Crops Res, 2004; 89:205–218.
- Lillemo M, Joshi AK, Prasad R, Chand R and Singh RP. Association of Lr34 and Lr46 with spot blotch resistance in wheat Theoretical and Applied Genetics, 2012; 126: 711- 726.
- Rosyara UR, Subedi S, Duveiller E, and Sharma RC. Photochemical efficiency and SPAD value as indirect selection criteria for combined selection of spot blotch and terminal heat stress in wheat. Journal of Phytopathology, 2010; 158, 813–821.
- Sharma RC, Duveiller E, Ortiz-Ferrara G. Progress and challenge towards reducing wheat spot blotch threat in the Eastern Gangetic Plains of South Asia: is climate change already taking its toll. Field Crops Res, 2007; 103:109–118.
- Miller P, Lanier W and Brandt S. Using growing degree days to predict plant stages. Montana state University, 2001; MT200103AG 7/2001: 1-8.
- Chaurasia S, Chand R, and Joshi AK. Relative dominance of Alternaria triticina Pras.et Prab.and Bipolaris sorokininana (Sacc.) Shoemaker, in different growth stages of wheat (T. aestivum L.). Journal of Plant Disease & Protection, 2000; 107, 176–181.
- Yao Q, Zhou R, Fu T, Wu W, Zhu Z, Li A and Jia J. Characterization and mapping of complementary lesion mimic genes lm1 and lm2 in common wheat. Theor. Appl. Genet, 2009; 119: 1005-1012
- Kumar U, Joshi AK, Kumar S, Chand R, and Röder MS. Mapping of resistance to spot blotch disease caused by Bipolaris sorokiniana in spring wheat. Theoretical and Applied Genetics, 2009; 118, 783–792.
- Zadoks JC, Chang TT and Konjak CF. A decimal code for the growth stages of cereals. Weed Research, 1974; 14, 415–421.
- Saari EE and Prescott JM. A scale for appraising the foliar intensity of wheat disease. Plant Disease Reporter, 1975; 59:377-380.
- Li T and Bai G. Lesion mimic associates with adult plant resistance to leaf rust infection in wheat. Theor. Appl. Genet, 2009; 119: 13-21.
- Saghai- Maroof MA, Soliman KM, Jorgensen RA and Allard RW. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci USA, 1984; 81:8014-8018.
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |
| How to cite this article: Singh S, Mishra VK, Kharwar RN, Ahirwar RN, Sharma S, Yadav PS, Chand R: Distribution of Leaf Tip Necrosis Genes and its Association with Expression of Lesion Mimic Genes and Resistance to Spot Blotch in Spring Wheat. Int. J. Life. Sci. Scienti. Res., 2017; 3(1): 808-816. DOI:10.21276/ijlssr.2017.3.1.10 Source of Financial Support: UGC-CGIAR-CYMMIT, Conflict of interest: Nil |