ABSTRACT-
The zooplankton diversity was studied in four stations at Madduvalasa reservoir during June 2014 to May
’15 and forty five species were identified. Among eight groups, the diversity of Rotifera comprises of 17 species
(21.37%), Cladocera 8 (16.44%), Copepoda 5 (17.28%), Ostracoda 2 (15.21 %), Protozoa 3 (12.24%), Crustacea 9
(11.26%), Mollusca 1 species (01.60%) respectively along with fish larvae and eggs (04.61%). The monthly and group
wise zooplankton density analysed and found that the number was highest during summer followed by monsoon and
lowest during winter.
Key-Words- Zooplankton, Monthly variation, Madduvalasa reservoir, Rotifera, Copepoda
INTRODUCTION-
Plankton is one of the most favourable food items for many
aquatic organisms; almost all the fishes at their larval
stages depend on it and some of them exclusively feed on
zooplankton. They invariably form an integral component
for fresh water communities and contribute to biological
productivity [1]. In the last two decades, much attention has
been paid in tropical countries towards the study of biology,
ecology and toxicology of zooplankton due to their
important role in rapidly emerging concepts in
environmental management like Environmental Impact
Assessment (EIA). Zooplankton is good indicator of the
changes in water quality because they are strongly affected
by environmental conditions and respond quickly. The
study of zooplankton is necessary to evaluate the fresh
water reservoir in respect to their ecological and fishery
status [2]. The Zooplankton community fluctuates according
to physicochemical parameters of the environment,
especially Rotifer species change with biotic factors [3].
Zooplankton is the link between phytoplankton and fish;
hence, their qualitative and quantitative studies are of great
importance.
MATERIALS AND METHODS-
Study Area:
Sri Gorle Sriramulu Naidu Madduvalasa
reservoir is present at Madduvalasa village of Srikakulam
district, Andhra Pradesh, India (Fig. 1). Samples were
collected from four stations of the above reservoir i.e., S1:
Narendra puram, S2: Vangara, S3: Kottisa and S4:
Gudivada agraharam.
Fig. 1. Madduvalasa reservoir (18° 35' 30''N Latitude and 83° 37' 20'' E longitude)
Where,
N = Total no. of organisms/ lit of water filtered,
n = Number of organisms counted in 1 ml of sample,
v = Volume of concentrate plankton sample (ml),
V= Volume of total water filtered through (L)
The systematic identification of plankton was made by using standard keys of various authors [5-10].
Biodiversity: The statistical calculation on biodiversity of zooplankton was studied using the formula of Shannon- Wiener diversity index and Menhinick’s index [11-12] which was calculated as follows:
1: Shannon - Wiener diversity index
Shannon-Wiener index denoted by
H = -SUM [(pi) × ln(pi)]
SUM = summation
pi = proportion of total sample represented by species i Divide no. of individuals of species i by total number of samples
S = number of species = species richness
Hmax = ln(S) Maximum diversity possible
E = Evenness = H/Hmax
2: Menhinick’s index
Menhinick’s index (d1) = S / /ÖN Where,
d1 = Menhinick’s index
S = total number of species.
ÖN = total number of organism (density)
RESULTS-
In the present study, diversity and monthly availability of zooplankton in Madduvalasa reservoir are analyzed and given in Table 1. Forty five species were identified in four stations, which consist of rotifera, cladocera, copepoda, ostracoda, protozoa, crustacea, mollusca along with fish larvae and fish eggs.
Table 1. Check list of Zooplankton species at Madduvalasa reservoir, Srikakulam dt.
| Group | Family | Species |
|---|---|---|
| Rotifera | Brachionidae | Brachionus angularis (Gosse,1851) |
| Brachionus calyciflorus (Pallas, 1766) | ||
| Brachionus caudatus (Haner, 1937) | ||
| Brachionus diersicornis (Daday, 1883) | ||
| Brachionus plicatelis | ||
| Brachionus quadridentata (Hermann, 1783) | ||
| Keratella cochlearis (Gosse,1851) | ||
| Keratella tropica (Apstein, 1907) | ||
| Lecanidae | Lecane lunaris (Ehrenberg,1982) | |
| Lacane monostyla (Daday, 1897) | ||
| Gastropodidae | Gastropus minor (Rousselet 1892) | |
| Asplanchnidae | Ascomorpha ovalis (Begendal, 1892) | |
| Asplanchna sp | ||
| Synchaetidae | Synchaeta sp | |
| Polyarthra vulgaris (Carlin, 1943) | ||
| Philodinidae | Philodina citrine (Ehrenberg) | |
| Testudinellidae | Filinia longiseta (Ehrenberg) | |
| Cladocera | Daphnidae | Daphania pulex |
| Daphania carinata | ||
| Monia micrua (Kurz) | ||
| Monia brachiata | ||
| Bosminidae | Bosmina longirostris | |
| Chydoridae | Alona pulchella (King) | |
| Alona intermedia (Sars) | ||
| Alonella. Sp | ||
| Copepoda | Diaptomidae | Cyclopoid copepodite |
| Diaptomus pallidus | ||
| Cyclopidae | Cyclops sp | |
| Mesocyclops sp | ||
| Nauplius larva | ||
| Ostracoda | Cyprididae | Cypris sp |
| Stenocypris sp | ||
| Protozoa | Parameciidae | Paramecium caudatum |
| Vorticellidae | Vorticella campanula | |
| Epistylis sp | ||
| Crustacea | Prawn nauplius larva | |
| Zoea larva | ||
| Chironimid larva | ||
| Dragonfly nymph | ||
| Mayfly nymph | ||
| Damselfly nymph | ||
| Stonefly nymph | ||
| Waterbeetle nymph | ||
| Mosquito larva | ||
| Mollusca | Velligar larva | |
| Fish larvae | Fish larva | |
| Fish eggs | Fish eggs |
The monthly variation of zooplankton density (nos/ lit) at four stations found that the maximum number of rotifera (262 nos/lit) recorded at station 1 during May 2015 and minimum (142 nos/lit) at station 3 in November 2014. Followed by the maximum number of cladocera (186 nos /lit) recorded at station 1 occurred during May 2015 and the minimum (112 nos /lit) at station 2 in January 2015. The maximum number of copepoda (224 nos /lit) recorded at station 1 during May 2015 and minimum (120 nos/lit) at station 2 in December 2014. The maximum number of ostracoda (162 nos /lit) recorded at station1 during May 2015 and minimum (104 nos /lit) at station 4 in January 2015. The maximum number of protozoa (142 nos /lit) recorded at station 1 during May 2015 and minimum (75 nos /lit) at station 2 in January 2015. The maximum number of crustacea (132 nos /lit) recorded at station 2 during August 2014 and minimum (54 nos /lit) at station 4 in January 2015. The maximum number of mollusca (36 nos /lit) recorded at station 3 during August 2014 and the nil at summer season and the maximum number of fish larvae (66 nos /lit) recorded at station 2 during August 2014 and the number was minimum (18 nos /lit) at station 3 in May 2015 (Table 2).
Table 2. Monthly variation of zooplankton density (no. / lit) during June 2014 – May 2015
| STATIONS/ MONTHS | ROTIFERA | CLADOCERA | COPEPODA | OSTRACODA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | |
| JUN-14 | 236 | 204 | 222 | 216 | 162 | 152 | 158 | 148 | 158 | 145 | 152 | 155 | 158 | 142 | 148 | 142 |
| JUL | 215 | 195 | 201 | 198 | 145 | 142 | 136 | 146 | 158 | 133 | 128 | 142 | 148 | 138 | 144 | 143 |
| AUG | 195 | 181 | 187 | 196 | 136 | 128 | 138 | 122 | 146 | 138 | 135 | 138 | 136 | 132 | 138 | 136 |
| SEP | 172 | 169 | 159 | 178 | 125 | 118 | 127 | 116 | 143 | 142 | 138 | 142 | 132 | 128 | 132 | 138 |
| OCT | 163 | 172 | 168 | 158 | 122 | 115 | 120 | 122 | 128 | 126 | 132 | 124 | 128 | 125 | 126 | 128 |
| NOV | 150 | 146 | 142 | 148 | 132 | 130 | 125 | 134 | 138 | 134 | 128 | 134 | 122 | 112 | 126 | 125 |
| DEC | 178 | 153 | 163 | 169 | 144 | 125 | 132 | 138 | 124 | 120 | 134 | 128 | 112 | 108 | 115 | 118 |
| JAN-15 | 186 | 168 | 176 | 174 | 138 | 112 | 124 | 128 | 142 | 135 | 132 | 141 | 118 | 121 | 108 | 104 |
| FEB | 180 | 175 | 177 | 168 | 152 | 145 | 149 | 149 | 167 | 152 | 145 | 158 | 125 | 135 | 118 | 115 |
| MAR | 197 | 177 | 181 | 178 | 164 | 158 | 168 | 156 | 184 | 164 | 155 | 174 | 142 | 142 | 130 | 125 |
| APR | 205 | 196 | 188 | 204 | 178 | 166 | 172 | 175 | 202 | 187 | 164 | 192 | 158 | 158 | 142 | 136 |
| MAY | 262 | 244 | 254 | 237 | 186 | 175 | 178 | 184 | 224 | 198 | 188 | 198 | 162 | 156 | 155 | 146 |
| TOTAL | 2339 | 2180 | 2218 | 2224 | 1784 | 1666 | 1727 | 1718 | 1914 | 1774 | 1731 | 1826 | 1641 | 1597 | 1582 | 1556 |
| MEAN | 2240.25 | 1723.75 | 1811.25 | 1594.00 | ||||||||||||
| STATIONS/ MONTHS | PROTOZOA | CRUSTACEA | MOLLUSCA | FISH LARVAE | ||||||||||||
| S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | S1 | S2 | S3 | S4 | |
| JUN-14 | 126 | 115 | 122 | 118 | 077 | 122 | 092 | 078 | 022 | 032 | 026 | 028 | 058 | 062 | 048 | 055 |
| JUL | 131 | 112 | 125 | 124 | 122 | 126 | 115 | 098 | 032 | 025 | 032 | 028 | 042 | 057 | 045 | 064 |
| AUG | 118 | 106 | 105 | 108 | 124 | 132 | 124 | 112 | 025 | 033 | 036 | 024 | 064 | 066 | 038 | 068 |
| SEP | 102 | 108 | 100 | 115 | 122 | 128 | 118 | 121 | 028 | 034 | 025 | 022 | 052 | 060 | 046 | 052 |
| OCT | 090 | 092 | 085 | 102 | 105 | 122 | 122 | 104 | 025 | 022 | 024 | 022 | 048 | 056 | 033 | 047 |
| NOV | 082 | 085 | 092 | 096 | 102 | 113 | 108 | 096 | 012 | 016 | 021 | 019 | 042 | 056 | 032 | 042 |
| DEC | 095 | 098 | 086 | 091 | 102 | 108 | 110 | 086 | 0 | 009 | 014 | 017 | 038 | 053 | 025 | 035 |
| JAN-15 | 096 | 075 | 084 | 085 | 096 | 106 | 094 | 054 | 0 | 0 | 009 | 010 | 033 | 042 | 028 | 038 |
| FEB | 106 | 082 | 102 | 102 | 085 | 112 | 094 | 076 | 0 | 0 | 0 | 0 | 028 | 035 | 026 | 027 |
| MAR | 112 | 096 | 113 | 122 | 075 | 095 | 085 | 078 | 0 | 0 | 0 | 0 | 025 | 038 | 022 | 025 |
| APR | 135 | 102 | 126 | 131 | 062 | 077 | 082 | 065 | 0 | 0 | 0 | 0 | 022 | 032 | 021 | 019 |
| MAY | 142 | 118 | 135 | 138 | 065 | 082 | 077 | 072 | 0 | 0 | 0 | 0 | 022 | 027 | 018 | 022 |
| TOTAL | 1335 | 1189 | 1275 | 1332 | 1137 | 1323 | 1221 | 1040 | 144 | 171 | 187 | 170 | 474 | 584 | 382 | 494 |
| MEAN | 1282.75 | 1180.25 | 168.00 | 483.50 | ||||||||||||
The monthly group wise zooplankton diversity observed from June 2014 to May 15 at four stations (Table 3 & Fig. 2). Rotifera group in the present study observed to show a numeric superiority over other groups of zooplankton and occupied with 21.37%. Followed by copepod groups with 17.28%, cladocera with 16.44%, ostracoda with 15.21%, protozoa with 12.24%, crustacea with 11.26%, fish larvae and eggs with 4.61% and mollusca with 1.60%.
Table 3: Group wise zooplankton diversity during June 2014 – May 2015
| S. No | Groups | Number of organisms | Percentage (%) |
|---|---|---|---|
| 1 | Rotifera | 2240.25 | 21.37 |
| 2 | Cladocera | 1723.75 | 16.44 |
| 3 | Copepoda | 1811.25 | 17.28 |
| 4 | Ostracoda | 1594.00 | 15.21 |
| 5 | Protozoa | 1282.75 | 12.24 |
| 6 | Crustacea | 1180.25 | 11.26 |
| 7 | Mollusca | 168.00 | 01.60 |
| 8 | Fish larvae | 483.50 | 04.61 |
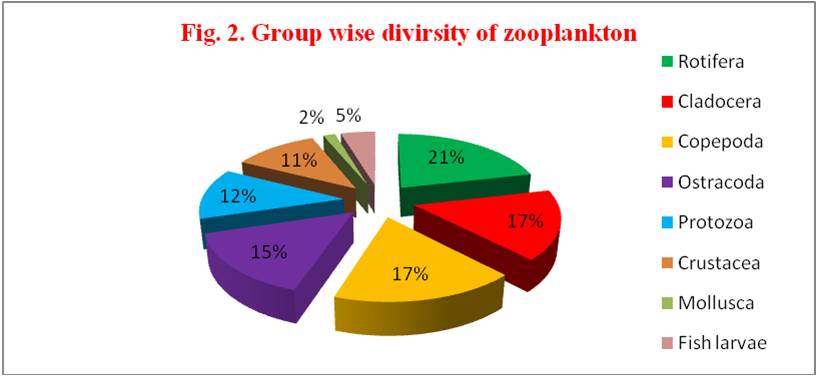
Fig. 2
Table 4. Monthly diversity of zooplankton during June 2014 to May 2015
| GROUP | SEASONS | |||||
|---|---|---|---|---|---|---|
| MONSOON | % | WINTER | % | SUMMER | % | |
| ROTIFERA | 781.00 | 34.86 | 653.50 | 29.17 | 805.75 | 35.97 |
| CLADOCERA | 549.75 | 31.89 | 510.25 | 29.60 | 663.75 | 38.51 |
| COPEPODA | 573.25 | 31.65 | 525.00 | 28.99 | 713.00 | 39.37 |
| OSTRACODA | 558.75 | 35.05 | 474.00 | 29.74 | 561.25 | 35.21 |
| PROTOZOA | 458.75 | 35.76 | 358.50 | 27.95 | 465.50 | 36.29 |
| CRUSTACEA | 452.75 | 38.36 | 407.00 | 34.48 | 320.50 | 27.16 |
| MOLLUSCA | 113.00 | 67.26 | 055.00 | 32.74 | 0 | 0 |
| FISH LARVAE | 219.25 | 45.35 | 162.00 | 33.51 | 102.25 | 21.15 |
Monsoon: Rotifera > Copepoda > Ostracoda > Cladocera > Protozoa > Crustacea > Fish larvae > Mollusca
Winter: Rotifera > Copepoda > Cladocera > Ostracoda > Crustacea > Protozoa > Fish larvae > Mollusca
Summer: Rotifera > Copepoda > Cladocera > Ostracoda > Protozoa > Crustacea > Fish larvae> Mollusca
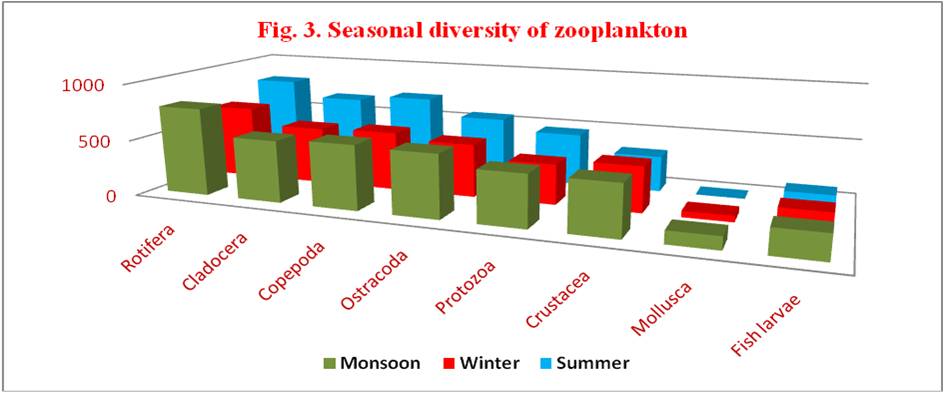
Fig. 3
Table 5. Group wise seasonal diversity of zooplankton during June 2014 to May 2015
| GROUP | MONSOON | % | WINTER | % | SUMMER | % |
|---|---|---|---|---|---|---|
| ROTIFERA | 781.00 | 21.07 | 653.50 | 20.78 | 805.75 | 22.19 |
| CLADOCERA | 549.75 | 14.83 | 510.25 | 16.22 | 663.75 | 18.28 |
| COPEPODA | 573.25 | 15.47 | 525.00 | 16.69 | 713.00 | 19.63 |
| OSTRACODA | 558.75 | 15.08 | 474.00 | 15.07 | 561.25 | 15.45 |
| PROTOZOA | 458.75 | 12.38 | 358.50 | 11.40 | 465.50 | 12.82 |
| CRUSTCEA | 452.75 | 12.22 | 407.00 | 12.94 | 320.50 | 08.82 |
| MOLLUSCA | 113.00 | 03.05 | 055.00 | 01.75 | 0 | 0 |
| FISH LARVAE | 219.25 | 05.92 | 162.00 | 05.15 | 102.25 | 2.82 |
| TOTAL | 3706.50 | 3145.25 | 3632.00 |
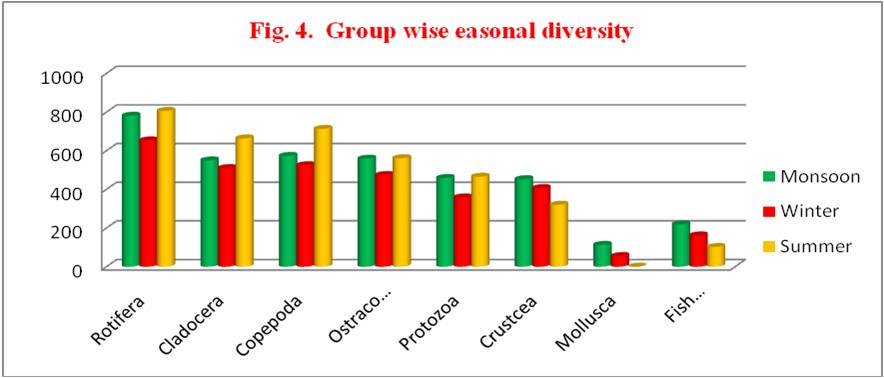
Fig. 4
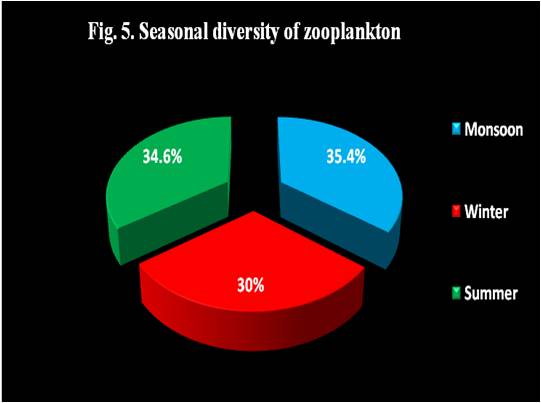
Fig. 5
Table 6. Shannon-Wiener and Menhinick’s diversity index
| Biodiversity Index | Monsoon | Winter | Summer |
|---|---|---|---|
| H= Shannon-Wiener Index | 1.98 | 1.95 | 1.82 |
| Hmax= Maximum diversity possible | 3.85 | 3.81 | 3.73 |
| E = Evenness | 0.51 | 0.51 | 0.49 |
| Menhinick’s index | 0.772 | 0.802 | 0.697 |
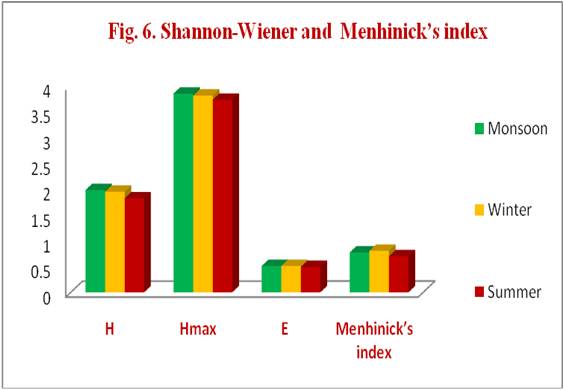
Fig. 6
In the present study, a total of 17 species of rotifer recorded from seven families on the whole, rotifera exhibited higher density in summer season. Rotifera play a vital role in the trophic tiers of fresh water impoundments and serve as living capsule of nutrition [18]. Sharma and Diwan [19] reported rotifera to form a dominant group during summer in Yeswinisagar reservoir; similar results were occurred at various fresh water bodies in India [20-24]. In the present investigation the population density of rotifera found rich in summer season (805.75 nos/lit) and less in winter season (653.50 nos/lit). A similar study was conducted on the number of rotifera which increased in summer may be due to the higher population of bacteria and organic matter of dead and decaying vegetation [7, 25-26]. According to Hutchinson [27], Brachionus species are very common in temperate and tropical waters indicating alkaline nature of water and excess growth of rotifera and reservoirs indicate the eutrophic conditions.
In the present study period, a total of 8 species of cladocera were recorded in three families. The population densities of cladocera were highest in summer season (663.75 nos/lit) followed by monsoon (549.75 nos/lit) and lowest in winter (510.25 nos/lit.). Diversity has also been reported higher in summer and lower in winter in Thigra Reservoir Gwalior [28] and in Majalgaon reservoir [24], cladocera is an order of small crustacea commonly called as “water fleas”. It has been reported that the density and biomass of cladocera was primarily determined by food supply [29]. Jhingran [30] recorded cladocera population to be most abundant in February, followed by July and Oct. in Ramgarh reservoir in Rajasthan. Sharma and Diwan [19] studied plankton dynamics of Yeshwantsagar reservoir in which the cladocera showed maximum density in June. Khare [31] observed an increasing trend in the months of winter season with peak during summer months March to June.He recorded minimum population during rainy season. Five species of copepoda from two families were recorded during the present study period. Copepoda showed higher population density in summer season (713 nos/lit) and lowest in winter (525 nos/lit). Similar results have also been reported to various seasonal fluctuation of zooplankton [24, 32-34]. In the present investigation two species of ostracoda were recorded from one family showing higher population diversity in summer season (561.25 nos/lit) and lowest in winter (474 nos/lit). Rajkumar [24] also reported 2 species of ostracoda a very low diversity and population density as compared to other groups of zooplankton. The population density was higher in summer season (851 org/lit) and less in Monsoon (637 org/lit). The similar results have also been observed various water bodies at different districts in India [35-37].
Three species of protozoa from two families were recorded during the present study. The density of population is highest during summer season (465.50 nos/lit) and lowest in winter season (358.5 nos/lit). Rajkumar [24] reported two species of protozoa and population density was higher in summer season (590.333 org/lit) and less in monsoon (379.333 org/lit). Similar observation was made by Shivashankar [38] at Bhadra reservoir, Karnatka. In the present investigation crustacea, mollusca, fish larvae and fish eggs play a vital role in the reservoir. The crustacea leads to sixth position in total number of organisms which comprises nine species like prawn nauplius larva¸ zoea larva, chironimid larva, dragonfly nymph, stonefly nymph, waterbeetle nymph, mosquito larva contain 11.26% in the total population. Importance of phytoplankton in Kalyanapulova reservoir was reported by Sasikala [39].
CONCLUSION- In the present study the seasonal variation in the diversity and distribution of zooplankton in Madduvalasa reservoir in all eight groups of zooplankton were recorded throughout the study period. The number was highest during summer and lowest during winter seasons in this reservoir. Shannon-wiener and Menhinicks biodiversity indices have been indicated that the zooplankton was evenly distributed in all seasons in Madduvalasa reservoir. It provides more information than simply the number of species present in four stations by revealing the abundance of rare and common species in different seasons.
REFERENCES
- Kanagasabhapati V and Rajan MK. A Preliminary survey of plankton in Irrukkangudi reservoir, Virudhnagar District, TN, India. Journal of Phytology, 2010, 2 (3): pp 63-72.
- Goswami, A.P., Mankodi, P.C. (2012). Study on zooplankton of freshwater reservoir Nyari- II Rajkot Dist, Gujarat, India, ISCA. J. Biol. Sci., 1(1): 30 34.
- Karuthapandi M, Rao DV and Xavier Innocent B. Zooplankton composition and diversity of Umdasager, Hyderabad. Int. J. Life Sci. Edu. Res. 2013; 1(1): 21 26.
- APHA. Standard methods for examination of water and waste water. 20th edn., American Public Health Association, Washington, D.C. ,1998.
- Pennak R.W. Freshwater Invertebrates of United states, 2nd Ed., John Wiley and Sons New York. 1968; pp 1-803.
- Krishnaswamy S. A Guide to the study of freshwater organisms.1973.
- Adoni AD, Joshi G, Gosh K, Chowasia SK, Vaishy AK, Yadav M and Verma HG. Work book on limnology, Prathibha Publishers, Sagar, India, 1985.
- Dhanapathi M.V.S S.S. Taxonomic notes on the Rotifera from India (from 1889-2000). Indian association of Aquatic Biologists’ (IAAB), Hyderabad. 2000.
- Altaff K. A manual of Zooplankton. Department of Zoology, the New College, Chennai. University Grants commission, New Delhi.2004.
- Lynne M Witty. Practical Guide to Identifying Freshwater Crustacean Zooplankton 2nd edition 2004; 1-49pp
- Shannon, C.E. and W. Weaver. The Mathematical Theory of Communication. University Illinois Press, Urbana. 1963; 1-117.
- Menhinick Edward F. A Comparison of Some Species- Individuals Diversity Indices Applied to Samples of Field Insects. Ecology. 1964; (45), 859–861.
- Ramachandrarao R and Mukundarao S. Checklist and economic classification of fresh water fishes of the Madduvalasa reservoir in Palakonda division, Srikakulam district, A.P. India. Int. J Fauna and Biological studies. 2015; 2(1): 25-29.
- Kudari VA, Kanamadi RD & Kadadevaru GG. Limnological studies of Attiveri and Bachanki reservoir of Utar Kannada district, Karnataka, India, Ecology, Environment and Conservation. 2005; 13(1): pp 1-6.
- Naveed, Md.S., A. Saboor and K.Altaff . Studies on the planktonic fauna of Madhavaram pond. Poll.Re. 2005; 24 (spl. issue): 199-204.
- George J P. “Limnological Investigations on the Plankton of Govindgarh Lake and Co-relation With Physico Chemical Factors”, Proc. Semi. Ecol. Fish Fresh Water Reservoir, 1970; pp. 37-46.
- Vasanth K. B. Khajure P. V. and Roopa, S.V. zooplankton and bacterial diversity in three ponds of Karwar District, Karnataka. Rec. Res. Sci. Tech. 2011; 39-48.
- Suresh Kumar, Altaff, R.K. and Raghunathan, M.B. New record of a Chydorid Cladoceran, pleuroxuy Aduncus jurine (1920), from Chennai, South India, with the description of the Development stages, International Journal of Aquatic Biology. 1999; 14 (1& 2), pp 7-10.
- Sharma Rekha and Diwan AP. Limnological studies of YeshwantSagar Reservoir Plankton population dynamics. Recent Advances in freshwater Biology, Ed. K.S. Rao, 1993; 1:199-211.
- Deshmukh U.S. Ecological studies of Chhatri Lake, Amravati with special reference to plankton and productivity. Ph. D. thesis Amravati University, Amravati. 2001.
- Akin-oriola G.A. Zooplankton association and environmental factors in Ogupa and Ona rivers, Nigeria. Rev. Biol. Trop. 2003; (2):391-398.
- Kadam S.U., Gaikwad J.M. and Md. Babar. Water quality and ecological studies of Masoli Reservoir in Parbhani District, Maharashtra. Ecology of Lakes and Reservoir Ed. V.B. Sakhare, Daya Publishing House, Delhi. 2006; pp 163-175.
- Rajashekhar M., Vijaykumar K. and Paerveen Zeba. Seasonal variations of Zooplankton community in freshwater reservoir Gulberga District, Karnataka, South India. Int. J. of Systems Biology. 2010; (1):6-11.
- Rajkumar T. Pawar. Zooplankton diversity and seasonal variation of Majalgaon reservoir, Maharashtra state, India. Int. J. Envi. Sci. 2016; 6:5.
- Segers H. A biogeographical analysis of rotifera of the genus Trichocerca Lamarck, 1801 with notes on taxonomy, Hydrobiologia. 2003; 500, pp 103-114.
- Majagi G. and Vijaykumar K. Ecology and abundance in Karanja reservoir. Environ. Monit. Asses. 2009; 152: 137-144.
- Hutchinson G. E. A treati se on Limnology, Vol. II: Limnoplancton. Wiley. New York.1967; 1015.
- Dushyant kumar Sharma and R.P Singh (2012), seasonal variation in zooplankton diversity in Tighra Reservoir Gwalior (M.P.) Indian Journal of Science and Research, 3(2), pp 155-161.
- Smitha P.G., Byrappa K. and Ramaswamy S.N. Physico chemical characteristics of water samples of bantwal Taluk, South-estern Karnataka, India, Journal of Environmental Biology. 2007; 595.
- Jhingran A.G. Limnology and production biology of two man-made lakes on Rajasthan (India) with management strategies for their fish yield optimization. Final Report IDA Fisheries Management in Rajasthan. Central Inland Fisheries Research Institute, Barrackpore, India: 1989; 1-63.
- Khare P.K. Physico-chemical characteristics in relation to Abundance of plankton of JagatSagar Pond, Chattapur, India. Advances in Limnology Edited by S.R. Mishra (Daya Publishing House), NewDelhi. 2005; pp 162-174.
- Somani V. and Pejavar M. Crustacean zooplankton of Lake Masunda, Thane, Maharashtra, International Journal of Aquatic Biology. 2004; l (19), pp 57-60.
- Mustapha M. K. Zooplankton assemblage of Oyun reservoir, Offa, Nigeria. Rev. Biol. Trop, International Journal of Tropical Biology. 2009; 57 (4), pp 1027-1047.
- Mahor R. K. Diversity and seasonal fluctuation of zooplankton in freshwater reservoir Tighra Gwalior (M.P.), Internet Referred Research Journal. 2011; 1(17), pp 47-48.
- Sukand B.N. and Patil H.S. Water quality assessment of Fort lake of Belgaum (Karnataka) with special reference to zooplankton, Journal of Environmental Biology. 2004; 25(1), 99-102.
- Kedar G.T., Patil G.P. and Yeole S.M. Effect of physicochemical factors on the seasonal abundance of zooplankton population in Rishi Lake. Proceedings of Taal 2007. The 12th world lake conference. 2008; pp 88-91.
- Patil S D, and Shirgur G A Morphology and identification characteristics of copepod species occurring in the government fish farm, Goregaon, Mumbai, Journal Eco biology, 2004; 16(1), pp 45-52.
- Shivashankar P and Venkataramana GV. Zooplankton diversity and their Seasonal varation in Bhadra Reservoir Karnatka, India. 2013.
- Sasikala T, Manjulatha C and D.V.S.N.Raju. Freshwater phytoplankton communities in Varaha reservoir, Kalyanapulova, Visakhapatnam. International journal of zoology studies. 2016, 1(5):5-7.
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |
| How to cite this article: Ramachandra RR, Manjulatha C, Raju D V S N: Zooplankton Diversity in Madduvalasa Reservoir, India. Int. J. Life. Sci. Scienti. Res., 2017; 3(1): 771-778. DOI:10.21276/ijlssr.2017.3.1.4 Source of Financial Support: Nil, Conflict of interest: Nil |