ABSTRACT-
Background & Objectives: Fungal infections of the eye are rampant in tropical regions and are
responsible for significant ocular morbidity and blindness. Prompt and specific identification of fungi is important in order
to define clinical treatment. However, in most cases conventional culture identification can be considered to be time
consuming, of low sensitivity and not without errors. The aim of the study is to evaluate a polymerase chain reaction
(PCR) based assay for fungal infections in the eye.
Methods: Polymerase chain reaction (PCR) for detection of fungal DNA was done with panfungal primers (ITS1 and
ITS4) for diagnosis of fungal aetiology. Ocular samples (conjunctival swabs, corneal scrapings, corneal biopsies, vitreous
aspirates and globe contents) from 30 patients with presumed fungal infection were evaluated using this assay, as well as
by standard microbiological techniques, and the results were compared.
Results: Thirty clinical specimens were evaluated by PCR and by other conventional techniques (culture and staining). Of
the 30 specimens analyzed, fungal keratitis was definitively diagnosed by culture in 18 (60%). 17 (57%) of these 18
specimens were PCR positive. One specimen (3% of the 30 total) was fungal culture positive but PCR negative. 12 (40%)
of 30 specimens were fungal culture negative, and 7 (23%) of these 12 were also PCR negative. Five (17%) were PCR
positive but fungal culture negative. Of the seven specimens negative by both PCR and fungal culture, all showed no
growth.
All 30 specimens were also examined by light microscopy with potassium hydroxide (KOH) and Gram’s staining. KOH
and/or Gram’s staining was found to be positive in 8 (27%) out of 30 cases. All positive smears revealed septate hyphae. 3
(38%) of these 8 specimens showed fungi on smear but were fungal culture negative, and two of these three specimens
were PCR positive which were clinically to have fungal keratitis.
Conclusions: PCR not only proved to be an effective rapid method for the diagnosis of fungal infections in eye but was
also more sensitive than staining and culture methods. Due to promising results of the DNA extraction protocol and PCR
assay in our laboratory, we were able to make early diagnosis. Hence, effective therapy was given, which had a major
impact on the improvement in the prognosis of subject with fungal infections.
Key-Words- Fungal keratitis, Lyticase, Proteinase K, Polymerase chain reaction, PCR assay, DNA extraction
INTRODUCTION-
Fungal infections of the eye are on rise and are responsible
for ocular morbidity and blindness.Fungal infections of the cornea (mycotic or fungal keratitis,
keratomycosis) are usually present as suppurative, usually
ulcerative, lesions [1]. Fungal keratitis in particular occurs
most frequently in individuals who work in agriculture
[2-3]. This condition is also associated with diabetes
mellitus [4] and the acquired immune deficiency syndrome
(AIDS) [5].
The incidence of invasive fungal infections has increased
dramatically in recent years. Early and specific diagnosis is
vital, and the decision to treat a patient with invasive fungal
infections is based mainly on clinical and mycological
information. However, the traditional methods (culture and
microscopy) used in routine practice for the diagnosis of invasive fungal infections may be insensitive and
somewhat nonspecific [6]. This has focused attention on
the rapid and accurate diagnosis of invasive fungal
infections using molecular biological techniques. These
methods have the potential to provide both high detection
rates and identification of specific fungal pathogens, as the
latter becomes increasingly important with the widespread
use of antifungal therapy and the problem of antifungal
resistance. The use of molecular diagnostic tools to detect
fungal specific nucleic acid sequences has recently been
reviewed [7-9], and many researchers have reported the
usefulness of DNA-based methods for the diagnosis of
invasive fungal infections. However, a major limitation of
the molecular method in comparison to blood culture was
the difficulty associated with problems in breaking fungal
cell walls since the DNA extraction step is still a limiting
factor, requiring more than half of a working day.
A novel pan ocular DNA extraction method was
standardized without using commercial DNA extraction kit,
which involved enzyme lyticase and proteinase K having
greater activity to disrupt cell wall of moulds. Polymerase
chain reaction (PCR) for detection of fungal 28S ribosomal
DNA was done with panfungal primers (ITS1 and ITS4)
was performed to detect the fungal genome in the ocular
fluids of patients with fungal keratitis. Ocular samples
(conjunctival swabs, corneal scraping, corneal biopsies,
vitreous aspirate, foreign body and globe contents) from
patients with presumed fungal infection were evaluated
using this assay, as well as by standard microbiological
techniques, and the results were compared. Hence, it was
concluded that PCR not only proved to be an effective
rapid method for the diagnosis of fungal infections in eye
but was also more sensitive than staining and culture
methods.
MATERIALS AND METHODS-
Patient Selection and Sample Collection-
The present study was conducted on the samples of
suspected infectious keratitis patients attending the out
(OPD) and indoor patient department (IPD) of
opthalmology at SRMS IMS, Bareilly, India from Nov
2015 to Mar 2016. The samples were processed at Central
Research Laboratory, Department of Biochemistry at
SRMS IMS, Bareilly. Ethical approval was not needed for
the current study as all samples were received for the
clinical diagnosis.
A common protocol for diagnosis was used in all cases
included in the study. Conjunctival swab was taken on a
sterile swab-stick passed across the lower fornix. Corneal
scrapings were obtained from clinically suspected cases of
fungal corneal ulcers by the corneal surgeon using a sterile
surgical blade no. 15 mounted on a Bard-Parker handle
under topical anaesthesia (0.5% proparacaine
hydrochloride) and slit lamp magnification. Corneal
biopsies were the excised corneal tissues from patients
undergoing therapeutic penetrating keratoplasty for fungal
corneal ulcers. Vitreous aspirates from clinically and sonologically proven cases of infective (presumably fungal)
endophthalmitis following intraocular surgery or trauma
were also included for the study. These were obtained from
eyes having endophthalmitis before giving intravitreal
injections and from eviscerated globe contents in case of
panophthalmitis, in the operation theatre. A total of 30
patients were included in the study.
Conventional Microbiological Investigations-
Direct microscopic examination at X400 and X1000
magnification of the ocular samples was performed with
10% KOH wet mount and/or by Gram's staining for
demonstration of fungal elements by a trained
microbiologist. Another portion of the collected sample
was inoculated directly on culture media such as blood agar
(BA), chocolate agar (CA), Brain-heart infusion broth
(BHIB) and Sabouraud's dextrose agar (SDA) without
cycloheximide. This inoculation was done in a C pattern. A
small portion from the blades used for scraping from the
cornea, or the syringes in which vitreous aspirates were
collected, was added to microfuge tubes to be processed for
PCR analyses. BA, CA and BHIB were incubated for 1
week at 37°C and were examined daily and discarded after
7 days if no growth or turbidity was seen. SDA was
incubated at 25°C and 37°C for 4 weeks. Cultures were
checked daily during the first week and twice a week for
the subsequent 3 weeks. Any growth obtained was further
identified by standard laboratory techniques.
The cultures were considered positive if at least one of the
following criteria was fulfilled:
1.The growth of the same organism was demonstrated on
one or more solid media and/or if there was
confluent growth at the site of inoculation on at least one
solid medium.
2.The growth on one medium was consistent with direct
microscopic findings.
3.The same organism was grown from repeated corneal
scrapings in suspected fungal keratitis.
DNA Extraction for PCR Optimization-
DNA Extraction from Culture-
To standardize the fungal DNA extraction for filamentous
fungi, vitreous fluid that was collected from eyes given
intravitreal injections of antibiotics but which showed no
infective growth on bacterial or fungal culture, was
artificially seeded with different concentrations of A. flavus
(American Type Culture Collection, Rockvilla, Maryland,
ATCC 90028) achieving 100, 101, 102, 103, 104, 105
conidia/ml. DNA extraction from 300 µl volume of spiked
specimen (150 µl vitreous fluid and 150 µl conidial
suspension in NET (.15 M NaCl, 10 mM EDTA and 10
mM Tris pH 7.5) buffer was performed according to the
method described by Skladny et al. [10] for BAL samples.
However, in the first step the spiked sample was incubated
for 90 min at 37°C with lyticase rather than 30 min. The
following steps of DNA extraction were done without any
alteration. DNA pellet was dissolved in 30 µl of Tris EDTA (TE) buffer.
PCR Standardization-
PCR was done with panfungal primers ITS1 (IDT,
Belgium) (5'TCC GTA GGT GAA CCT GCG G'3), and
ITS4 (IDT, Belgium) (5' TCC TCC GCT TAT TGA TAT
GC 3') to amplify ITSI, 5.8 S and ITSII region of the
rDNA. The 50 µl PCR mixture contained 10 µl of DNA
template, 5 µl PCR buffer with 1.5 mM MgCl2, 200 µM
each deoxynucleoside triphosphate (100 µM Bangalore
Genei, Bangalore), 25 pmol of each primer, and 1.5 U of
Taq DNA polymerase (Himedia, Mumbai). Reaction
involved 1 cycle at 95°C for 5 min, followed by 35 cycles
with a denaturation step at 95°C for 30 sec, an annealing
step at 55°C for 1 min, and an extension step at 72°C for 1
min, followed by 1 cycle of 72°C for 6 min (done in XP
thermal cycler, Bioer, Japan). Candida albicans (ATCC
204303), Aspergillus flavus (ATCC 90028), Fusarium
solani (clinical isolate), Staphylococcus aureus (ATCC
25923), Escherchia coli (ATCC 25922), Enterococcus
faecalis (ATCC 29212) and Pseudomonas aeruginosa
(ATCC 27853) were included as control in the present
study. Ten microliter of the PCR products, mixed with
loading buffer was then electrophoresed 1.5% agarose gel
with 0.5 µg/ml ethidium bromide. A 100 base pair (bp)
DNA ladder (Himedia, Mumbai) was used for determining
the size of the amplicons. The gels were visualized using
ultraviolet illumination. Images were captured and stored
by using a UVP Gel Documentation System (UVP Ltd,
USA).
Extraction of DNA from Ocular Samples-
Swabs from conjunctival sac, corneal scrapings, corneal
biopsies and globe contents were added into a microfuge
tube containing 300 µl of NET buffer. The same method of
DNA extraction as followed for culture was performed
without any alteration in the clinical samples. Ten
microliter of DNA extracted was added to PCR mixture
and the PCR reaction was performed following the standard
protocol. Positive control (DNA of A. flavus ATCC 90028)
and negative control (bacterial genomic DNA of S. aureus
ATCC, E. coli ATCC, E. faecalis, P. aeruginosa and
without any template DNA) were carried out
simultaneously. Aliquots (10 µl) of the PCR product was
analysed on a 1.5 % agarose gel as described above.
RESULTS AND DISCUSSION-
Standard Fungal Isolate-
PCR Specificity:
The primers used in this study (ITSI
and ITS4) successfully amplified DNA from all the
standard fungal strains tested. A product of approximately
500-600 bp was obtained. No amplification products were
detected by using ITS1 and ITS4 primer pair with S.
aureus, E. coli, E. faecalis, and P. aeruginosa.
PCR Sensitivity:
Using cell dilution, the PCR was
positive with samples containing 10 conidia/ml of A. flavus (Fig. 1). Detection of amplified product in these spiked
samples indicated that no PCR inhibitors were present after
DNA extraction.
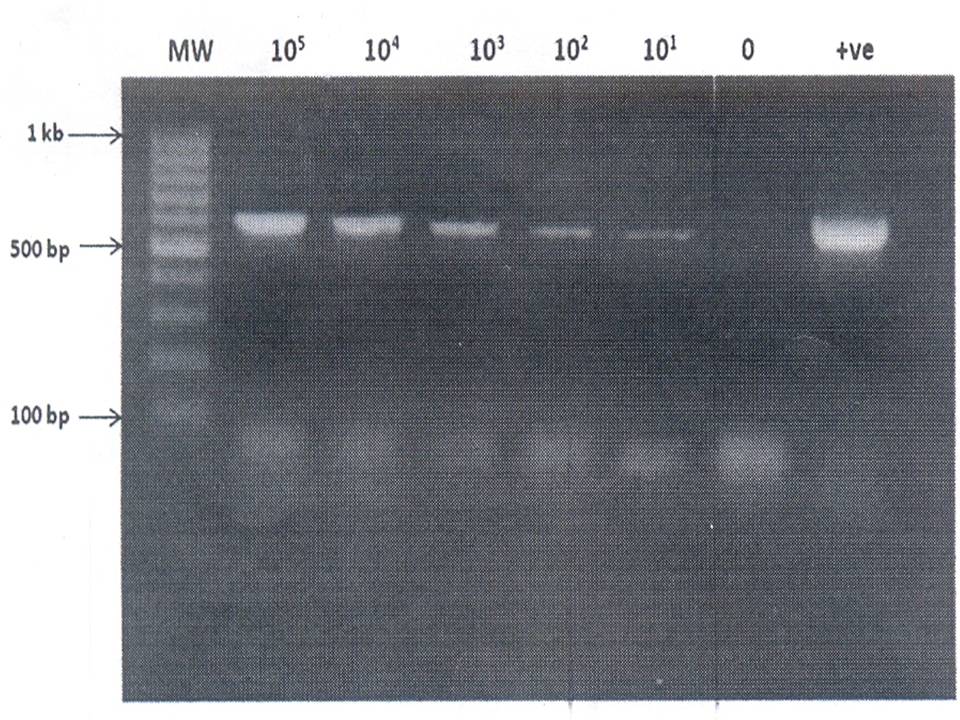
Fig. 1: Agarose gel electrophoresis of amplified fungal DNA extracted from Spiked samples
Lane 2-7: 105 to 100 conidia of A. flavus/ml
Lane 8: Positive control (A. terreus DNA)
Ocular samples- Thirty clinical specimens were evaluated by PCR and by other conventional techniques (culture and staining). The results are shown in Table 1. Of the 30 specimens analysed, fungal keratitis was definitively diagnosed by culture in 18 (60%). 17 (57%) of these 18 specimens were PCR positive (Fig. 2). One specimen (3% of the 30 total) was fungal culture positive but PCR negative- an apparent “false negative” PCR result. Twelve (40%) of 30 specimens were fungal culture negative, and seven (23%) of these 12 were also PCR negative.
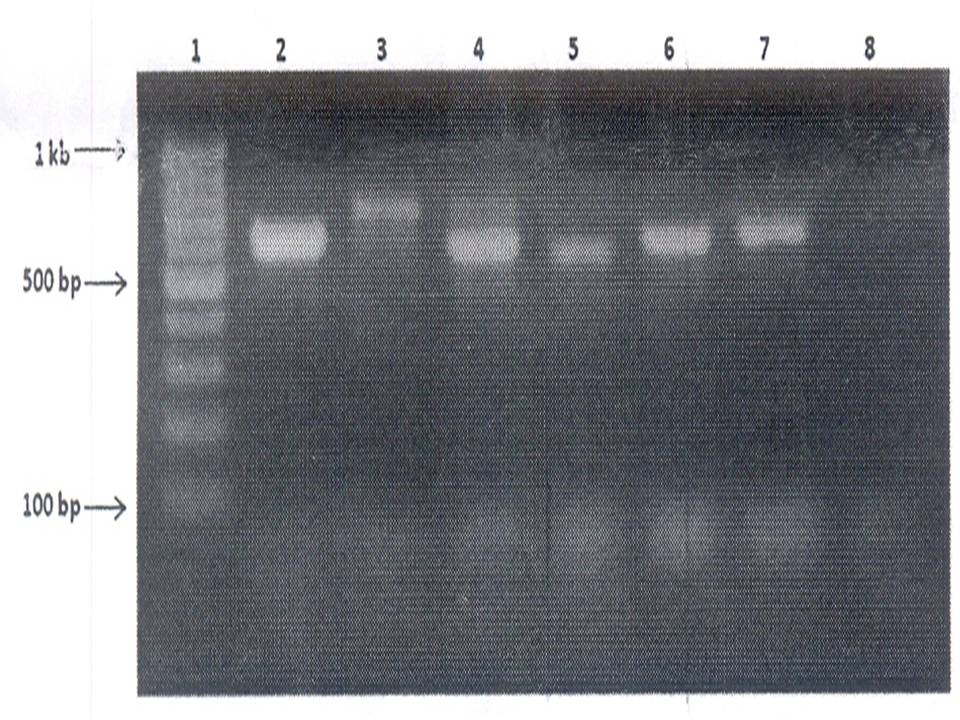
Fig. 2: Agarose gel electrophoresis of amplified fungal DNA extracted from Clinical samples
Lane 2-6: Clinical samples
Lane 7: Positive control (A. terreus DNA)
Lane 8: Negative control
Five patients were PCR positive but fungal culture negative (Table 1); their clinical charts were reviewed. Based on the result of fungal staining and their response to antimicrobial treatment, 2 (7%) patients appeared clinically to have fungal keratitis despite negative fungal culture results. 2 (7%) patients were judged clinically to have bacterial infections, and one patient was lost to follow up with an uncertain clinical course. Among the 18 culture positive specimens (Table 1), six (20%) harboured Fusarium in culture, two (7%) had Aspergillus, and 10 (33%) culture isolates were not speciated. No specimen was found in culture to harbour yeast, and no specimen was positive with only C. albicans primers. Of the seven specimens negative by both PCR and fungal culture (Table 1), all showed no growth.
Table 1: Results of culture and Polymerase Chain Reaction analysis of samples from patients with presumed infectious keratitis
| N=30* | Culture positive for fungi | Culture negative for fungi |
|---|---|---|
| PCR positive for fungi | 17 (57%) Culture results: Fusarium: 6 (20%) Aspergillus: 2 (7%) Unidentified: 10 (33%)± | 5 (17%) Clinical findings: Fungal keratitis: 2 (7%) Bacterial keratitis: 2 (7%) Uncertain: 1 (3%) |
| PCR negative for fungi | 1 (3%) Culture result: unidentified± Clinical finding: fungal keratitis | 7 (23%) Culture results: No growth: 7 (23%) |
| 18 (60%) | 12 (40%) |
All 30 specimens were examined by light microscopy with potassium hydroxide (KOH) and Gram’s staining. KOH and/or Gram’s staining was found to be positive in 8 (27%) of 30 cases. All positive smears revealed septate hyphae. Three (38%) of these 8 specimens showed fungi on smear but were fungal culture negative, and two of these three specimens were PCR positive which were clinically to have fungal keratitis.
DISCUSSION- In this report, we present our experience in the detection of fungal pathogens in ocular samples to diagnose fungal keratitis. This study demonstrates that fungi can be detected in infected corneas using PCR techniques. The advantage of PCR as shown is its rapidity. The PCR assay used in this study required 6 hours to generate results, significantly faster than the 2 days to 1 week required for any fungal culture technique in confirming the culture growth. On the other hand fungal smears also provide result quite quickly, hence most clinicians and microbiologists resort to direct microscopic examination of the Gram’s smear and KOH wet mount for rapid diagnosis. But its effectiveness is variable and results are not definite. Another advantage of PCR based assay is its high sensitivity over conventional methods, ability to be performed in scanty samples and/or dead microorganisms present in the ocular samples.
A key parameter influencing the usefulness of a PCR assay in a clinical setting is a good DNA extraction method from fungi is essential before the amplification of DNA. Many earlier protocols used the enzyme Zymolyase (Novozyme 234, ICN Pharmaceuticals, Costa Mesa, CA, USA; or Mureinase, United States Biochemicals Corp. Cleveland, OH, USA) to disrupt the fungal cell wall. This results in the formation of fungal spheroplasts which have increased osmotic sensitivity [11]. Zymolyase efficiently release DNA from yeasts such as Candida and Crytococcus spp. [12], but it is ineffective in disrupting the cell walls of moulds, including Aspergillus spp. [13]. To overcome this obstacle, mechanical disruption with heat alkali treatment [14] or with a combination of glass beads and repeated freeze thawing using liquid nitrogen [15] have been used. However, these methods have some drawbacks like shearing of DNA, low sensitivity of the protocol due to inefficient fungal cell wall disruption and inefficient DNA release etc. However, another ß-1, 3-glucanase, lyticase (Sigma) has been shown to effectively generate spheroplasts in moulds [16]. Lyticase has greater activity against the cell wall of moulds [17].
With the above parameters in mind, we required a DNA extraction protocol which should be sensitive enough to detect fungi in scanty amount of samples from eye and should cause minimal loss of shearing of DNA. Hence enzymatic method (combination of lyticase and proteinsae K) for DNA extraction was selected [10]. This protocol was evaluated in Bronooalveolar lavage (BAL) and blood samples [10] and again in blood and tissues [18]. In our study, we not only evaluated the efficacy of the DNA extraction protocol in spiked samples but also tried its applicability in various ocular samples without using commercial DNA extraction kit.
The patients included were clinically diagnosed as fungal ocular infections, 29 patients having corneal ulcers and 6 with endophthalmitis or panophthalmitis. Patients with corneal ulcer were clinically diagnosed to be having fungal infection on basis of suggestive history like that of trauma with vegetative matter, long duration of symptoms, history of previous treatment, etc, and because of morphological features like marked ciliary congestion, presence of slough over the ulcer surface, dry appearance, feathery margins, satellite lesions and hypopyon. Of the other patients, 4 presented with delayed postoperative endophthalmitis, 1 with presumed endogeneous endophthalmitis and 1 with post-traumatic panophthalmitis. Those with endophthalmitis were known diabetic patients with poor or borderline control and presented with endophthalmitis a few weeks to months after intraocular surgery. One patient with endogenous endophthalmitis was suffering from septicaemia post septic abortion. One young male required evisceration due to panophthalmitis following penetrating ocular trauma that showed no intraocular foreign body on radiological screening.
In this study, the sensitivity and specificity of PCR was found to be 94% and 58% respectively double to that of standard culture methods for detecting fungi in ocular samples.
CONCLUSION- We concluded in this study that ocular fungal infections are increasing and gaining attention in clinical practice. Therefore, newer research focused on improvement in diagnostic techniques with cost efficiency would be immensely helpful to the ophthalmologist in decreasing the morbidity associated with ocular mycosis. Hence, this method will be useful for fungal infections irrespective of ocular samples in developing countries like India where the diagnostic test should be cost and time effective. However, there is a need to evaluate this protocol in larger number of patients with fungal infection in the eye.
ACKNOWLEDGMENT- The authors thank SRMS IMS Trust, Bareilly for providing the platform for this research work Authors also thank Dr Arunaloke Chakrabarti, Professor, PGIMER, Chandigarh for providing standard fungal cultures and Dr. Neelima Mehrotra, Opthalmologist/Professor SRMS Hospital, Bareilly for providing the ocular samples.
REFERENCES
- Thomas, PA. 2003. Fungal infections of the cornea. Eye, 17: 852–862.
- Deshpande, SD, Koppikar, GV. 1999. A study of mycotic keratitis in Mumbai. Indian Journal of Pathology and Microbiology, 42: 81–87.
- Johnson, GJ, Minassian, DC, Weale, R. 1998. The epidemiology of eye disease. 1st ed. New York: Chapman and Hall Medical, 143.
- Ormerod, LD, Hertzmark, E, Gomez, DS et al. 1987. Epidemiology of microbial keratitis in southern California. A multivariate analysis. Ophthalmology, 94:1322–1333.
- Mselle, J. 1999. Fungal keratitis as an indicator of HIV infection in Africa. Tropical Doctor, 29: 133–135.
- Malhotra, S, Sharma, S, Bhatia, NJK, et al. 2014. Recent Diagnostic Techniques in Mycology. Journal of Medical Microbiology and Diagnosis, 3: 146.
- Reiss, E, Tanaka, KG, Bruker, V, et al. 1998. Molecular diagnosis and epidemiology of fungal infections. Medical Mycology, 36: 249-257.
- Vazquez, JAS. 1994. Use of the polymerase chain reaction for the diagnosis of invasive Candida infection. Serodiagnosis and Immunotherapy in Infectious Disease 6: 173-178.
- Walsh, TJ and Chanock, SJ. 1998. Diagnosis of invasive fungal infections: advances in nonculture systems. Current Clinical Topics in Infectious Diseases 18: 101-153.
- Skladny, H, Buchheidt, D, Baust, C, et al. 1999. Specific detection of Aspergillus species in blood and bronchoalveolar lavage samples of immunocompromised patients by two-step PCR. Journal of Clinical Microbiology, 37(12): 3865–3871.
- Kitamura, K, Kaneko, T, Yamamoto, Y. 1974. Lysis of viable yeast cells by enzymes of Arthrobacter luteus. II. Purification and properties of an enzyme, zymolase, which lyses viable yeast cells. Journal of General and Applied Microbiology, 20: 323–344.
- Varma A, Kwon-Chung KJ. 1991. Rapid method to extract DNA from Cryptococcus neoformans. Journal of Clinical Microbiology, 29: 810– 812.
- Reiss, E, Obayashi, T, Orle, K, et al. 2000. Non-culture-based diagnostic tests for mycotic infections. Medical Mycology, 38: S147–S159.
- Loeffer, J, Hebart, H, Sepe, S, et al. 1998. Detection of PCR-amplified fungal DNA by using a PCR-ELISA system. Medical Mycology, 36: 275–279.
- Hopfer, RL, Walden, P, Setterquist, S, et al. 1993. Detection and differentiation of fungi in clinical specimens using polymerase chain reaction (PCR) amplification and restriction enzyme analysis. Journal of Medical Veterinary Mycology, 31: 65–75.
- Van Burik, JA, Myerson, D, Schreckhise, RW, et al. 1998. Panfungal PCR assay for the detection of fungal infection in human blood specimens. Journal of Clinical Microbiology 1998; 36: 1169–1175.
- Scott, J, Schekman, R. 1980. Lyticase: endoglucanase and protease activities that act together in yeast cell lysis. Journal of Bacteriology, 142: 414–423.
- Nguyen, TH, Nguyen, DHC, Phong, PT, et al. 2012. Extraction of Human Genomic DNA from Dried Blood Spots and Hair Roots. International Journal of Bioscience, Biochemistry and Bioinformatics, 2: 21-26.
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |
| How to cite this article: Kaur J, Singh J, Mishra P: Polymerase Chain Reaction Based Detection of Fungi in Suspected Infectious Keratitis Patients. Int. J. Life. Sci. Scienti. Res., 2017; 3(1): 766-770. DOI:10.21276/ijlssr.2017.3.1.3 Source of Financial Support: Nil, Conflict of interest: Nil |