Key-Words- Larvicidal, Medicinal plant extracts, Phytochemicals Analysis, Culex quinquefasciatus
INTRODUCTION- The mosquito is the principal vector of many of the vector borne diseases affecting human beings and other animals. Several mosquito species belonging to genera Anopheles, Culex and Aedes are vectors for the pathogens of various diseases like malaria, filariasis, japanese encephalitis, dengue fever, dengue hemorrhagic fever and yellow fever. Repeated use of synthetic insecticides for mosquito control has disrupted natural biological control systems and led to resurgences in mosquito populations. It has also resulted in the development of resistance, undesirable effects on non-target organisms and fostered environmental and human health concern, which initiated a search for alternat-ive control measures. Plants are considered as a rich source of bioactive chemicals and they may be an alternative source of mosquito control agents One of the approaches for control of these mosquito borne diseases is the interruption of disease transmission by either killing, preventing mosquitoes to bite human beings (by using repellents) or by causing larval mortality in a large scale at the breeding centers of the vectors. This study is concerned with the using of such effective plant source against the larval of Mosquito. Blood-feeding female mosquitoes are responsible for the intolerable biting nuisance and transmission of a large number of diseases such as malaria, yellow fever, dengue, filariasis, chiken-gunya, and en-cephalitis. They cause serious health problems to humans and present obstacles to the socioeconomic development of developing countries, particularly in the tropical region [1]. In addition to mortality, vector borne diseases cause morbidity of millions of persons resulting in loss of man-days and causing economic loss [2].
There are 300 species of mosquitoes belonging to 41 genera; all contained in the family Culicidae.
Aedes aegypti, a vector of dengue is now endemic and found to be widely distributed in the tropic and subtropics. Synthetic chemical larvicides are parts of the world. But many of these chemicals are toxic to human, plant and animal life and insecticides resistance can be problematic in maintaining control, especially with organophosphate and pyrethroid larvicides [3]. Therefore, a more efficient approach to reduce the population of mosquitoes would be to target the larvae.
Mosquitoes are ecologically important components of the aquatic and terrestrial food chain, then they are the most important group of insects in terms of public health importance, and thus, appropriate control programs are jus-tified. Until a few years ago, only the adults were sprayed, but now, it is well known that a more efficient way to reduce mosquito populations is to target the larvae [3-4].
MATERIALS AND METHODS-
The list of plant leaves included in this study was
1. Vitex negundo (Nochi)
2. Azadirachta indica (neem)
3. Eucalyptus tereticornis (Thailam)

Fig. 1: Medicinal plants
Preparation of Medicinal Plant Extracts- 10 grams of each plant leaves powder was taken separately and 100ml of solvents (Ethanol, Petroleum ether, Hexane and Acetone extract)was added and kept overnight in the shaker. The extract is filtered again the solvent is added and kept at room temperature in the shaker for 7 hours. In this way the plant extract is collected and stored in the air tight container for future use.
Test for Carbohydrates- 1ml of different crude extract was dissolved in 10ml of distilled water and filtered. The filtrate underwent Mol-ish’s test to confirm the presence of carbohydrates [5].
Molish’s Test- The filtrate was treated with 2ml of concentrated sulphuric acid along the sides of the test tube. Appear-ance of violet colour ring at the junction of the two liquid shows the presence of carbohydrates.
Test of Glycosides- A small portion of different crude extract was hydrolyzed with hydrochloric acid for few hours on water bath and the hydrolysate was collected [5].
Fehling’s Test- 2ml of extract was taken in a test tube. 1ml of Fehling’s A solution and 1ml of Fehling’s B solution was added to the extract, mixed well and boiled. Appearance of yellow or red colour precipitate indicates the presence of glycosides (reducing sugar).
Test for Proteins- A small portion of crude was dissolved in few ml of distilled water and it was subjected to Xantho protein test to confirm the presence of protein [5].
Xantho Test- Take 3ml of extract to which 1ml of concentrated nitric acid was added. A white precipitate was obtained. The solution was heated for 1 minute and cooled under the tap water. 40% of NaOH was added to the solution. Appearance of orange colour indicates the presence of protein.
Test for Phenolic compounds and Tannins- A small portion of crude extract was dissolved in few ml of distilled water and subjected to FeCl3 test [5].
FeCl3 Test- Few ml of extract 5% FeCl3 was added. Appearance of violet colour indicates the presence of phenolic compounds and tannins.
Test for Flavonoids- The crude extract was treated with concentrated sul-phuric acid. Appearance of yellowish orange colour indicates the presence of anthocyanin, on further adding yellow turns to orange which indicates the pres-ence of flavones, on further adding turns to crimson which indicates the presence of flavonones [5].
Test for Terpenoids- 2ml of crude extract was dissolved in 2ml of chloro-form to which 2ml of sulphuric acid was added and the heated for 2 minutes. Appearance of grayish colour indicates the presence of terpenoids.
Test for Phlobatannins- 2ml of extract was taken to which 2ml of 1% HCl was added and boiled. Formation of red precipitate indicates the presence of phlobatannins [5].
LARVICIDAL BIOASSAY- Initially 12.5 mg crude extract of petroleum ether solvent of each solvent extract was taken and dissolved in 1ml of acetone in an eppendorf. Then the dissolved crude extract was mixed in container containing 50 larvae in 100ml of water. Every 24 hours the mortality rate was noted and reading was taken. The dead larvae were taken out at every 24 hours since it may leads to contamination of the water. The readings were taken for 2 days (48hours) then 0.25% (250ppm) concentration from each plant crude extract was introduced into containers containing larvae. Similarly for this reading wastaken for every 24 hours for 2 days. Then 0.50% concentration (500ppm) of crude extract was introduced and reading was noted for every 24hrs for two days [6].
Qualitative Analysis of Phytochemicals- The phyto constituents detected in the plant extract could be responsible for their antioxidant and antimicrobial activity.
Table 1: Qualitative analysis of phytochemicals in various extracts of Vitex negundo
| Phytochemicals/ Extracts | Ethanol extract | Petroleum ether extract | Hexane extract | Acetone Extract |
|---|---|---|---|---|
| Alkaloids | - | + | - | + |
| Steroids | + | + | + | - |
| Triterpenoids | + | - | - | + |
| Flavanoids | + | + | + | + |
| Tannins | - | - | - | + |
| Phenols | + | + | - | - |
| Glycosides | + | - | + | + |
| Saponins | - | - | - | - |
| Phlobotannins | + | + | + | - |
| Anthraquinones | + | + | - | + |
(-): absence of phytochemicals
Table 2: Qualitative analysis of phytochemicals in various extracts of Azadirachta indica
| Phytochemicals/ Extracts | Ethanol extract | Petroleum ether extract | Hexane extract | Acetone Extract |
|---|---|---|---|---|
| Alkaloids | - | + | - | + |
| Steroids | + | + | + | - |
| Triterpenoids | + | - | - | + |
| Flavanoids | + | + | + | + |
| Tannins | - | - | - | + |
| Phenols | + | + | - | - |
| Glycosides | + | - | + | + |
| Saponins | - | - | - | - |
| Phlobotannins | + | + | + | - |
| Anthraquinones | + | + | - | + |
(-): absence of phytochemicals
Table 3: Qualitative analysis of phytochemicals in various extracts of Eucalyptus tereticornis
| Phytochemicals/ Extracts | Ethanol extract | Petroleum ether extract | Hexane extract | Acetone Extract |
|---|---|---|---|---|
| Alkaloids | - | + | - | + |
| Steroids | + | + | + | - |
| Triterpenoids | + | + | - | - |
| Flavanoids | + | + | + | + |
| Tannins | - | - | - | + |
| Phenols | + | + | - | + |
| Glycosides | + | + | + | + |
| Saponins | - | + | - | + |
| Phlobotannins | + | + | + | - |
| Anthraquinones | + | - | - | + |
(-): absence of phytochemicals
RESULT AND DISCUSSION-
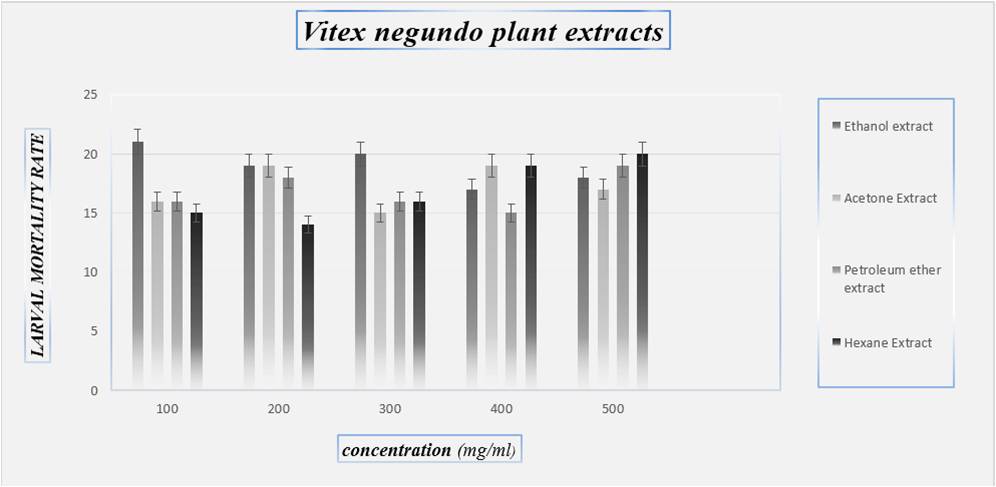
Fig 2: Larvae mortality rate on Vitex negundo plant
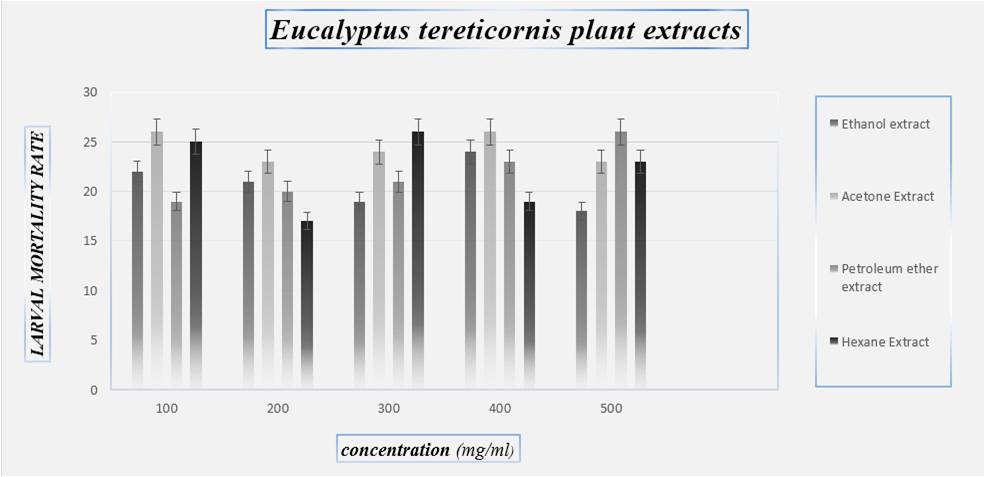
Fig 3: Larvae mortality rate on Eucalyptus tereticornis plant
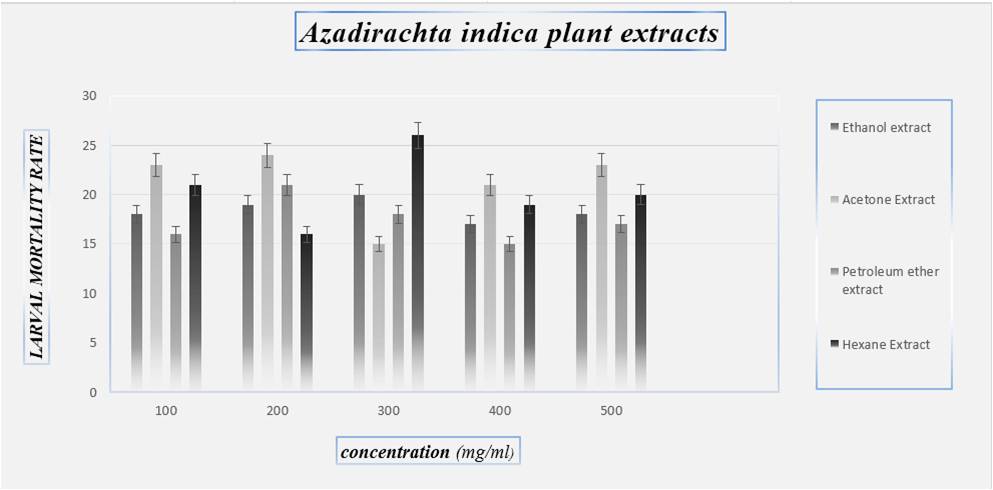
Fig 4: Larvae mortality rate on Azadirachta indica plant
CONCLUSION- An insecticide does will not cause high mortality on target larvae but may serve medicinal plants extracts as suitable alternatives to synthetic insecticides .plant extract are more safer, inexpensive, reduce dependence on imported products(synthetic) and are readily available in many region of the world. The findings of the present investigation revealed that Eucalyptus tereticornis has good larvicidal activity against Culex quinquefasciatus and Azadirachta indica showed poor mortality against the Culex quinquefasciatus larvae. These crude extracts can be effectively used in the control of mosquitoes by replacing the chemical pesticides which cause environmental pol-lutions and other burdens. Statistically Vitex negundo had high larvicidal efficacy. These Medicinal plants extracts are the best alternate product against the control of mosquito’s vector. It can be replace the chemical pesticides which cause environmental pollutions and other health problems.
REFERENCES
- Senthilkumar A, Kannathasan K, Venkatesalu V. Chemical constituents and larvicidal property of the essential oil of Blumeamollis (D. Don) Merr against Culex quinquefasciatus. Parasitol Res 2008; 103: 959-962.
- Dhiman RC, Pahw S, Dhillon GP, Dash AP. Climate change and threat of vector-borne diseases in India: are we prepared? Parasitol Res 2010; 106(4): 763-773.
- Rahuman AA, Bagavan A, Kamaraj C, Saravanan E, Zahir AA, Elango G. Efficacy of larvicidal botanical extracts against Culex quinquefasciatus Say (Diptera: Culicidae). Parasitol Res 2009; 104(6): 1365-1372.
- Govindarajan M, Jebanesan A, Pushpanathan T. Larvicidal and ovicidal activity of Cassia fistula Linn. Leaf extract against filarial and malarial vector mosquitoes. Parasitol Res 2008; 102(2): 289-292.
- Arun J, Pennarasi, Prasanna. M, SundaraMahalingam, Narendhiran, Alagesan, Esaipriyan. Screening of Phytochemical Constituents towards Antimicrobial Potency of Hygrophilaschulli. A Journal of Biotechnology. 2231-3826, Special Issue 2013
- Maheswaran, R., Sathish. S and Ignacimuthu. S. 2008. Larvicidal activity of Leucasaspera against the larvae of Culex quinquefasciatus and Aedes aegypti. International Journal of Integrative Biology, 2(3):214-217.
- Su T, Mulla MS. Ovicidal activity of neem products (azadirachtin) against Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J Am Mosq Control Assoc 1998; 14:204-9.
- Sukumar K, Perich MJ, Boobar LR. Botanical derivatives in mosquito control: a review. J Am Mosq Control Assoc 1991;7 : 210-37.
- Dhar R, Dawar H, Garg S,Basir SF, Talwar GP. Effect of volatiles from Neem and other natural products on gonotrophic cycle and oviposition of Anopheles stephensi and An. culicifacies (Diptera: Culicidae). J Med Entomol 1996; 33:195-201.
- Su T, Mulla MS. Effects of Neem products containing azadirachtin on blood feeding, fecundity, and survivorship of Culex tarsalis and Culex quinquefasciatus (Diptera: Culicidae). J Vector Ecol 1999; 24:202-15.
- Lucantoni L, Giusti F, Cristofaro M, Pasqualini L, Esposito F, Lupetti P, et al. Effects ofneem extract on blood feeding, oviposition and oocyte ultrastructure in Anopheles stephensi Liston (Diptera: Culicidae). Tissue Cell 2006; 38: 361-71.
- Mulla MS, Su T. Activity and biological effects of Neemproducts against arthropods of medical and veterinary importance. J Am Mosq Control Assoc 1999; 15: 133-52.
- Boschitz C, Grunewald J. The effect of NeemAzal on Aedesaegypti (Diptera: Culicidae).ApplParasitol 1994; 35: 251-6.
- Nathan SS, Kalaivani K, Murugan K. Effects of Neemlimonoids on the malaria vector Anopheles stephensi Liston (Diptera: Culicidae). Acta Trop 2005; 96: 47-55.
- World Health Organization. Instruction for determining thesusceptibility or resistance of mosquito larvae to insecticides.Geneva: WHO/VBC/81.807; 1981.
- Singh KRP, Patterson RS, LaBrecque GC, Razdan RK. Mass rearing of Culex pipiensfatigans Wied. J Commun Dis 1975;7 : 31-53.
- Brown MD, Thomas D, Watson K, Kay BH. Laboratory and field evaluation of efficacy of vectobac 12AS against Culex sitiens (Diptera: Culicidae) larvae. J Am Mosq. Control Assoc1998; 1: 183-5.
- Abbott WS. A method of computing the effectiveness of an insecticide. J Econ Entomol 1925; 18: 265-7.
- Finney DJ. Probit analysis, 3rd ed. Cambridge: Cambridge University Press; 1971.
- Sun C, Georghiou GP, Weiss K. Toxicity of Bacillus thuringiensis var israelensis to mosquito larvae variously resistant to conventional insecticides. Mosq News 1980; 40:614-8.
- Aly C, Mulla MS, Xu B, Schnetter W. Rate of ingestion by mosquito larvae (Diptera: Culicidae) as a factor in the effectiveness of a bacterial stomach toxin. J Med Entomol 1988; 25: 191-6.
- Aly C. Filtration rates of mosquito larvae in suspensions of latex microspheres and yeast cells. Entomol Exp Appl 1988; 46: 55-61.
- Azmi MA, Naqvi SNH, Ahmad I, Tabassum R, Anbreen B. Toxicity of Neem leaves extracts (NLX) “compare with Malathion” (57 E.C.) against late 3rd instar larvae of Culex fatigans (Wild Strain) by WHO method. Trop J Zool 1998; 22:213-8.
- Zebitz CPW. Potential of Neem seed kernel extracts in mosquito control. Proceedings of the third International Neem Conference (1986 Nairobi, Kenya) 1987. p. 555-73.
- El-Shazly MM, El-Sharnoubi ED. Toxicity of a Neem (Azadirachta indica) insecticide to certain aquatic organisms. J Egypt Soc Parasitol 2 000; 30: 221-31.
- Caboni P, Cabras M, Angioni A, Russo M, Cabras P. Persistence of azadirachtin residues on olives after field treatment. J Agric Food Chem 2002; 50: 3491-4.
- Caboni P, Sarais G, Angioni A, Garcia AJ, Lai F, Dedola F, et al. Residues and persistence of Neem formulations on strawberry after field treatment. J Agric Food Chem 2006; 54: 10026-32.
- Govindarajan M, Jebanesan A, Pushpanathan T, Samidurai K. Studies on effect of Acalypha indica L. (Euphorbiaceae) leaf extracts on the malarial vector, Anopheles stephensi Liston (Diptera: Culicidae). Parasitol Res 2008; 103(3): 691-695.
| Source of Financial Support: Nil Conflict of interest: Nil |
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/ |