Key-Words- Ubiquitous, Susceptibilities, Nosocomial infection, Surveillance
INTRODUCTION- Pathogenic fungi are fungi that cause disease in human or other organism. Fungi are ubiquitous in our environment. Fungal infections of hospital origin are gaining importance in recent years due to their progressive increase into the high rates of mortality and morbility with which they are associated [1-2]. Hospital–acquired infection or nosocomial infections are one of leading cause of death in the world and typically affect patients whose immune systems are compromised. This paper reports the result of environmental surveillance of fungi in specific areas of Private hospital of Jabalpur (M.P.) Air, dust and drinking water samples were collected from hospital. Many of these infections are endogenous in nature, but others can be acquired by exogenous roots, through the hands of healthcare workers,contaminated infusion products and bio-materials and abiotic environmental sources [3]. Candida species remain a major cause of nosocomial infection. Candida albicans was the main agent for causing Fungemia and Funguria [4]. [1] Reported that many of fungal infections arise from an endogenous source. Outdoor air markedly influences the prevalence of fungal spore levels in indoor air and thus, is the major source of fungal infections in indoor environments especially in hospitalized in-dividuals [5].
Mycotic diseases of man are an emerging public health problem which receives growing alternation from the health authorities. Aspergillus species are the common cause of hospital acquired fungal infection. Airborne spores probably also infect tissues exposed during surgery. Conidia of Aspergillus may gain entry, susceptible patient by contaminated hospital supplies [6-7]. [8] Isolated Aspergillus fumigates and A. Niger from the soil of various potted indoor plant kept in the room at the patient suffering from Aspergillosis. Aspergillums species were isolated in a multi centre hospital study from water mycoflora from the hospital environment [9]. In the hospital environment, the airborne micro biota is formed mainly by filamentous fungi, especially those belonging to the genera, Aspergillus, Cladosporium, Paecilomyces, Scopu Cariopsis [10]. Yeast has been found at the genera Candida, Rhodofforula, Uraptococeus and Trichosporon [11-13]. To identify the source of fungal infection the environment of the patient, viz. air, water and dust of the hospitalized room were investigated for the presence of pathogen. The pathogen were isolated in culture and identified on the basis of culture, micro and macro morphology.
MATERIALS AND METHODS-
Sampling Site: To explain the possible source of infection of deep mycoses, air, dust and water sample were screened from the environment of hospitalized patient were investigated. The air samples were collected from the General ward(G.W.), Private wards(P.W.), Deluxe room, Minor operation theater (MOT), Operation theater (OT), Intensive care unit (ICU). Dust sample were collected from the floor of passage of hospital and water sample was col-lected from the drinking water source of the hospital. At each location samples were taken at the time periods between 12:00 pm to 1:00pm which are visiting hours of the hospital. All the samples were taken during Year 2015.
Sample Collection:
A.Soil Sample Collection- Two number of Soil samples were collected from the floor of the Suvidha hospital, Jabalpur from April to June during the year 2015 in polythene bags. These samples were transported to the mycological laboratory and processed immediately.
(i) Processing of Samples- 1gm of air dried sieved soil was mixed with 10ml distilled water by vigorously shaking for was allowed to sediment for 5-10 minutes 5ml of the suspension was than kept at 37o C for 1 hour.
(ii) Sample Dilution- Petri plates sterilized at 60o C in an over for one day or autoclaved at 120o C at 15 lbs pressure were used for the isolation of fungi then dilution (1:10, 1:100, 1:000) of sample was prepared. In all the dilution of soil suspension, chlormphenicol was mixed (0.1 mg for each 5ml suspension) to inhibit bacterial growth. One ml each of all the three dilutions (1:10, 1:100 and 1:1000) was placed on previously prepared petri plates containing SDA media.
B.Method of Water Sample Collection- Water samples from the drinking water source of hos-pital were collected in sterilized plastic bottles. Three different dilutions (1:10, 1:100 and 1:1000) were prepared and processed further similar to as described under sample dilution from soil sample collection.
C.Method of Air Mycoflora Collection- Previously prepared SDA containing Petri plates was exposed at different corners of the general ward, private wards, deluxe room, Intensive care unit, minor operating theater, and operating theater of the hospital for 5–10 minutes. These Petri plates were then transported to the laboratory and kept in incubator up to 7 days at 37oC for the isolation of fungi.
Funguses were identified on the basis of their direct microscopic examination, Macro-micro morphological observation and Slide culture technique.
RESULT AND DISCUSSION- The air samples in the hospital yielded Aspergillus fumigatus, A. flavus, A. niger, A. terreus, Rhizopus sp. Fusarium sp., Microsporum sp., Alternaria sp. Tricophyton sp. Penicillium sp. and Candida sp (Table 1).
Table 1: List of Air Mycoflora
| S. No. | Ward | Yeast isolated | Moulds Isolated |
|---|---|---|---|
| 1 | General Ward | Candida sp. | Aspergillus niger, Aspergillus terreus, Aspergillus flavus, Aspergillus fumigates, Penicillium sp., Microsporum sp. Fusarium sp. |
| 2 | Private Ward Delux | Candida sp. | Aspergillus flavus Aspergillus niger, Tricophyton sp. |
| Private Ward1 | Candida sp. | Aspergillus flavus | |
| Private Ward2 | Nil | Aspergillus niger, Alternaria sp. | |
| 3 | Minor Operation Theater | Nil | Aspergillus fumigates, Aspergillus flavus, Aspergillus niger, Rhizopus sp., Penicillium sp. |
| 4 | Operation Theater | Nil | Aspergillus flavus, Alternaria sp., Fusarium sp., Aspergillus fumigates |
| 5 | Intensive care unit | Nil | Aspergillus flavus, Fusarium sp. |
The dust samples of floor of the hospital were positive for Aspergillus flavus, Aspergillus. Niger, Alternaria sp. and Fusarium sp. (Table 2).
Table 2: Dust Mycoflora
| S. No. | Sample No. | Yeast isolated | Moulds Isolated |
|---|---|---|---|
| (i) | Sample -1 | Nil | Aspergillus sp. Alternaria sp. |
| (ii) | Sample -2 | Nil | Fusarium sp. Asperigillus niger Aspergillus flavus |
The sample of drinking water of the hospital no fungi was isolated.
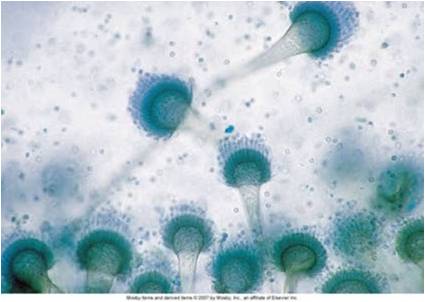
Fig 1. Aspergillus sp.
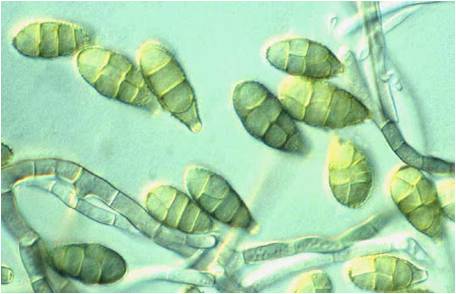
Fig 2. Alternaria sp.
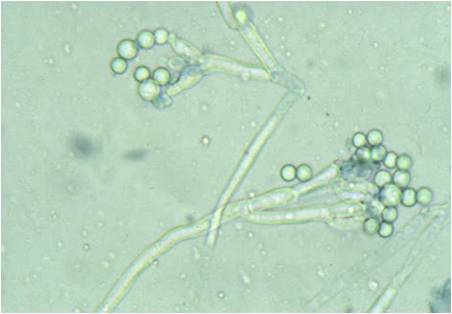
Fig 3. Penicillium sp.
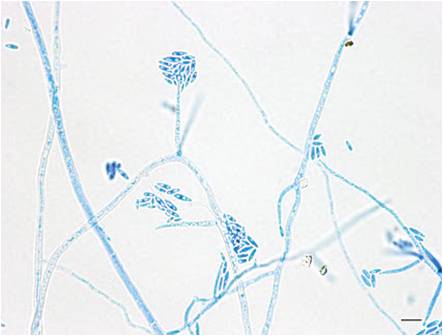
Fig 4. Fusarium sp.
In this study, the two investigated factors, namely the type of room and the time of sampling, individually or combined, were found to influence the microbial rate in indoor air of hospital infections. The result from this study showed that general ward had a higher degree of contamination with airborne fungi in indoor air. The factor which might be involved in the finding is the number of beds. The high bed number in general ward means a high number of patients, personnel and visitors occupying the ward and the multiple patients per room might raise the number of people in rooms and the corridors. These results indicate the kind of ward has a significant effect that influences the rate of airborne fungi in indoor air of the hospital. Intensive care units, the operation theater or neonatal wards considering the type of room, as a factor affecting the indoor rate of air-borne fungi, there was a significant effect of different levels of the degree of cleanness and disinfection strategies, which might lead to increased fungal rates in the patient room since this location is occupied with high number of people all of times, patients, visitors and personnel and lead to increase in-door rate of air borne fungi.
The large number of patients, visitors and personnel raise the microbial rates especially at the afternoon because of the maximum activity of people there. The exchange between indoor and outdoor air raise the microbial rate brought from outside the hospital into main entrance and this coincides the role of outdoor microbial concentration through opened windows and doors in raising the microbial rates. The fungal contamination in the operating room was extremely low. This was anticipated due to the high sanitary standards in this area, compared to the other hospital areas.
These airborne fungal species had been reported in several studied that used different isolation and identification procedures. These fungal pathogens are responsible for infection in immunocompromised patient. In general, the results of the present study clearly showed that airborne fungi from different groups are present as real contaminants of indoor air of the hospital. [14] Reported soil as a good source of C. albicans. The risk of invasive Aspergillosis mainly depends on the patient’s immune status [15]. [16] Isolated Candida spp., Aspergillus flavus, Aspergillus fumigates, Aspergillus niger, Aspergillus terreus, Rhizopus spp. and Fusarium spp. from environment of the patients like dust, water and air of the hospital wards [17].
CONCLUSION- Mycotic infections have increased significantly on the global bases and great public health problem in modern era. Fungal contamination in healthcare facilities has been the subject of numerous studies. Seven fungal genera Aspergillus, Alternaria, Candida, Fusarium, Rhizopus, Penicillium and Tricophyton were isolated and identified from indoor air of the time of visits in the Private hospital. The objective of study is to determine the fungal diversity and epidemiology causing discuses in Environmental of hospital patients. We conclude that General Ward had a higher degree of contamination with airborne fungi in indoor air and fungal contamination in the operating room was very low. It is further suggested that adherence to good infection control procedure, particularly hand washing, proper sterilization, use of air filter in the room of the hospitalized patients would definitely decreased the admittance of the number of such infections in the hospitals.
ACKNOWLEDGMENT- First and foremost we place on record of my gratitude to our esteemed guide Dr. Varsha Aglawe for her valuable gratitude and encouragement. We would also like to thank Doctors and staff of this hospital.
REFERENCES
- Colombo AL. Epidemiology and treatment of hematogenous candidiasis a Brazilian perspective. Braz J Infect Dis. 2000; 4: 113-8.
- Pfaller MA Nosocomial Candidiasis: emerging species, reservoirs and modes of transmission. Clin. Infect. Dis., 1996; 22 (2): 889-894.
- Wenzel RP. Nosocomial candidemia: risk factor and attributes mortality. Clin Infect Dis., 1995; 20: 1531-1534.
- Price DL, Simmons RB, Ezeonu IM, Crow SA, Abern DG. Colonization of fiber glass in solution used in heating, ventilation and air conditioning system. J. Ind. Microbiol, 1984; 13:154-158.
- Masoomeh SG, Sanaz AG, Narges A, Mehdi RA. Investigation on distribution of airborne fungi in outdoor environment in Tehran, Iran. Journal of Environmental Health Science and Engineering, 2014; 12: 54.
- Grossman ME. Primary cutaneous aspergillosis in six leukemic children. J. Am. Acad. Dermatol. 1985; 12: 313-318
- McCarty JM, Outbreak of primary cuteneous aspergillosis related to intravenous. Arm boards. J. Pediatr, 1986; 108: 213-218.
- Staib F. Ramuluffunter Suchung auf. Aspergillus Artch inder wohnung eines chronic lung enkranken. Bundesgesundhbl, 1978; 21: 471-474.
- Hayette MP, Christiaens G, Mutsers J, Barbier C, Huynem P, Melin P. Filamentous Fungi recovered from the water distribution system of a Belgian university hospital. Med Mycol, 2010; 48:969-974.
- Rainered J, Peintner U, Poder R. Biodiversity and concentration of airborne fungi in hospital environment. Mycopathologia, 2011; 149: 87-97.
- Krajewska K, Krajewska – Kulak E, Lukawzuk C, Rolka H, Lach J, Karczewski J. Occurrence of fungal pathogens in the delivery rooms of a hospital obstetrics department. Ginekol Pol, 2004; 75: 451-456.
- Pini G, Faggi E, Donato R, Fanci R. Isolation of Tricosporon in a haemotology ward. Mycoses. 2005; 48: 45-49.
- Moretti ML. A importancia crescente das infeccoes fungicas. Rev Panam Infectol, 2007; 8:8-9.
- Nawange SR. Search for ecological niche of human pathogenic fungi in the environment of Jabalpur with special reference to their pathogenic potentials. PhD thesis, 1999.
- Paugam A, Benchetrit M, Fiacre A, Tourte S, and Dupouy CJ. Comparison of four commercialized biochemical system for clinical yeast identification by colour producing reactions. Medical Mycology, 1999; 37:11-17.
- Aglawe V. Studies on deep mycoses with special reference to Etiology, Epidemiology, Serology and therapy. Ph.D. Thesis. 2004.
- Aglawe V, Mir AM, Patel S, Sontakke H. Isolation of opportunistic pathogenic fungal contamination from hospital environment. IJGSR, 2015; 2 (3):213-220.
| Source of Financial Support: Nil Conflict of interest: Nil |
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/ |