Key-Words- Channa straitus, Staphylococcus aureus, Hasanparthy, DLC, WBCs
INTRODUCTION- Aeromonas hydrophila,Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella salmonicida is the bacteria is responsible for red spot disease an important bacterial disease that occurs worldwide. It has a large economic impact on fish production because of the extensive and rapid rate of mortality that it causes [1-2]. The clinical signs of the disease begin with corrosion of the dorsal and tail fins, and this progresses to external infection in which gray spots or yellowed areas of erosion appear, generally surrounded by a hyperemic reddened zone, in the cranial region, body surface and gills.In these locations, there is progressive necrosis involving the epidermis, dermis and musculature [3]. India is a country where the favorable conditions exist for a wide diversity of fish production systems. However the major challenges are faced, particularly in relation to bacterial infections.
The Red spot disease (RSD) endemic to South and Southeast Asia, this is a serious disease of fresh water and estuarine fin fish [4] endemic to South and South-east Asia. It is a seasonal epizootic condition characterized by infection of Aeromonas hydrophila, Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella salmonicida and large he-morrhagic necrotizing ulcers typically producing a granulomatous response [5]. As a result of stocking density, ectoparasites, inadequate handling and stressful conditions, out breaks of motile Aeromonads associated diseases can reach epidemic proportions among the aquatic animals, leading to massive mortality rates [6]. There are several studies on fish bacterial identification and disease resistance [7-9]. Temperature is an essential and fluctuating environmental factor influencing all life activities which dominate remarkable changes on hematological parameters of aquatic animals. Knowledge of haematolgy is very important since it deals with the morphology, physiology and the biochemistry of fish blood [10]. The haematological parameters are important tools for diagnosis of healthy and infected fishes [11-12]. The blood analysis also reveals the disease status [13-14].
Warangal district of Telangana state has many lakes which include Hasanparthy and Dharmasagar lakes. These lakes are with different fishes with abundant C. striatus and C. punctatus with high quality of flesh and taste. They also have good market value due to low fat, fewer intramuscular spines and medicinal qualities [15]. The low winter temperature of 20-280C in this region is ideal for Red spot disease outbreaks which reduced the commercial value of this fish. The present study has taken up to the study of haematological changes in C. straitus and C. punctatuswith bacterial infected fishes were compared with control fishes.
MATERIALS AND METHODS-
The freshwater Channa straitus and Channa punctatus infected with different bacterial infection 200 fishes were collected irrespective of age, sex and size from, Hasanparthy and Dharmasagar lakes which are located at a distance of 30 Km from Kakatiya University campus during the rainy and winter seasons between June, 2013 to April, 2014. Blood samples were collected from caudal peduncle of both control and infected live fishes by dissecting in a tray separately. The collected blood was preserved in Di Potassium EDTA for further analysis as described by [16]. Red blood corpuscles (RBC) and White blood corpuscles (WBC) were counted by haemocytometer crystalline chamber using “Hayeman’s” diluting fluid. Haemoglobin (Hb) estimation was done by haemoglobinometer according to [17] and Differential leucocyte counts (DLC) were calculated by standard formulae, both in the control and bacterial and fungal infected fishes.
RESULTS AND DISCUSSION - The haematological study has become an essential tool for fishery research.It has been reported that the blood values remarkably vary in different fresh water fishes and this is considered to reflect adaptations to the various environmental conditions [10, 18]. The blood is a patho physiological reflector of whole body and therefore blood parameters are important in diagnosing the functional status of the an-imal exposed to toxicants. Anemia is also one of the most sensitive pathological situations developed as a result of metals poisoning [19]. (Table 1 & 2; Fig. 1 & 2), shows the results of haematological parameters such as RBC, WBC, HB and Differential Leukocyte Count.
Table. 1. Haematological paramaters of C. striatus (Bloch) infected with bacteria
| Blood Parameter | Control fish | Bacterial Infected fish | Percentage Change (%) | P value |
|---|---|---|---|---|
| HB (g%) | 11.3±0.34 | 8.7±0.31 | -91.3 | 0.0001 |
| RBCs (106/ml) | 4.4±0.25 | 1.96±0.14 | -98.04 | 0.0001 |
| WBCs (103/ml) | 41.67±947.51 | 50.37±893.85 | 50.27 | 0.0001 |
| Lymphocytes % | 52.0±0.71 | 57.4±0.80 | -42.6 | 0.0001 |
| Neutrophils % | 59.6±0.62 | 28.9±0.65 | -71.1 | 0.0001 |
| Monocytes % | 5.7±0.34 | 3.4±0.31 | -96.6 | 0.0001 |
| Monocytes % | 2.5±0.37 | 4.0±0.36 | -96 | 0.0106 |
| Basophils % | 2.3±0.26 | 1.7±0.35 | -98.3 | NS |
Table: 2. Haematological parameters of C. punctatus (Bloch) infected with bacteria
| Blood Parameter | Control fish | Bacterial Infected fish | Percentage Change (%) | P value |
|---|---|---|---|---|
| HB (g%) | 10.1±0.33 | 9.1±0.29 | -90.9 | 0.0373 |
| RBCs (106/ml) | 4.2±0.27 | 1.89±0.13 | -98.11 | 0.0001 |
| WBCs (103/ml) | 33.50±674.13 | 39.00±756.450 | 38.90 | 0.0001 |
| Lymphocytes % | 58.2±0.341 | 61±0.614 | -39 | 0.0009 |
| Neutrophils % | 34.8±0.64 | 29.3±1.67 | -70.7 | 0.0059 |
| Monocytes % | 5.8±0.37 | 4.4±0.74 | -95.6 | 0.0057 |
| Monocytes % | 2.6±0.37 | 4.1±0.38 | -95.9 | 0.0124 |
| Basophils % | 2.2±0.28 | 2.8±0.38 | -97.2 | NS |
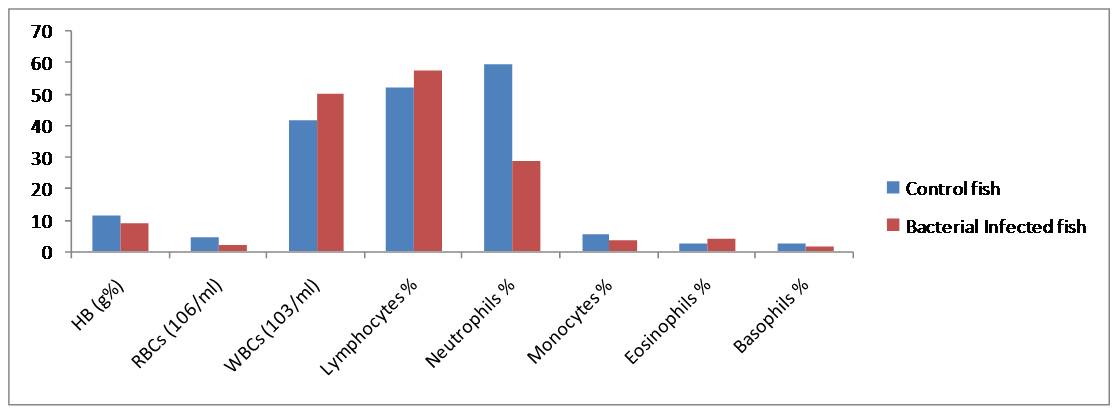
Fig. 1. Haematological responses in C. striatus showing % increase (+) or decrease (-) During the study period (2013-14) in Hasanparthy and Dharmasgar Lakes
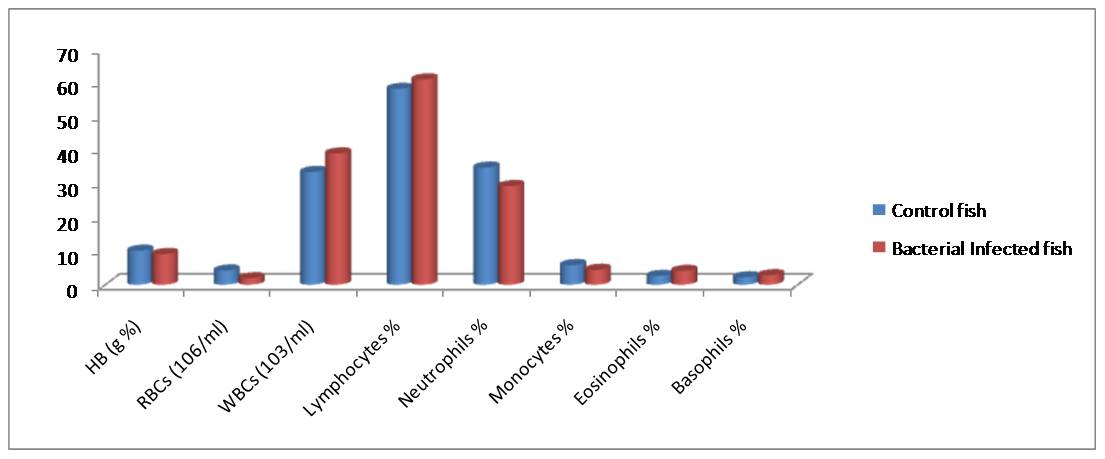
Fig. 2. Haematological responses in C. punctatus showing % increase (+) or decrease (-) During the study period (2013-14) in Hasanparthy and Dharmasgar Lakes
Haemoglobin (HB)- The hemoglobin content of Channa striatus and Channa punctatus were 11.3±0.34, grams and 10.1±0.33, grams percent in control fishes. Where as in fish infectedwith bacteria exhibited. It is 8.7±0.31grms and 9.1± 0.29, grms. In comparison with the control the HB % in infected fishes was found decreased by (23%) and (9%). The Hb variation in infected lakes of fishes was due to seasonal pollution, low oxygen, heavy native fishes and chemical stress [20-21]. Have also reported a decrease of Hb content in fish Heteroneustes fossils exposed to paper mill effluents. The bacterial infection influences the malfunctioning of hematopoietic system [22]. Corresponding to the decrease of RBC count due to the bacterial infection, the hemoglobin percentage was also exhibited a similar decrease in fishes.
Red Blood Corpuscles (RBC)- The results have showed a significant decrease in RBC number. The Total Erythrocyte count was observed to be decreased, in Red spot disease (RSD) infectious Channa striatus and Channa puntatus by (63.5%), (55%) in relation to control. A decrease in RBC was also reported [23]. Which is sedentary habit of these fishes, the bacterial infection induced extravasation of blood and reduction of haemo-synthesis which in turn fails the hematopoietic tissue to release the blood cells [22]. Erythrocytes or the red blood corpuscles are the major cellular elements found in the blood. The observations revealed that the structure of normal red cell or normocyte varies in shape from being oval or eliptical, biconcave disc or round (Fig. 3 & 4).
Fig. 3 Erythrocytes (Control)
Fig. 4 Erythrocytes (Infected)
Total Leucocyte Count (TLC)- A significant increase (21%) and (17%) of total leucocyte count were foundin the infected fishes. The enormous increase of leucocyte count in infected C. striatus and C. punctatus could be correlated to leuococytosis due to anoxic stress, low temperature and lack of food, high organic and inorganic compounds (NH3) and heavy native fishes [24]. While studying on C. striatus and C. punctatus have reported that leucocytes constitute second important category of the circulating blood cells and will show increased proliferation on exposure to mitogen, concanavalin, Aeromonas hydrophila, Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella salmonicida infections indicates the leucocytes are polyclonally activated (Fig. 5 & 6).
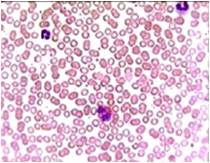
Fig. 5 Luecocytes (Control)
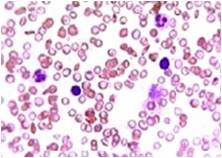
Fig. 6 Luecocytes (Infected)
Lymphocytes- Present study reveals that the lymphocyte percentage in the control C. striatus and C. punctatus fishes were 52.0±0.71% and 58.2± 0.341%. Whereas a significant increase of (5.4%) and (5%) was recorded in the infected fishes. This condition is called as the lymphocytosis which is an immunological response against the stress induced by the polluted environment. An increase of lymphocytes indicates the pathological condition due to bacteria and stress full toxic substances in the infected fish [25]. An enhanced lymphocyte proliferation was also observed in Atlantic Manhadevan with ulcer disease syndrome [26] (Fig.7 & 8).
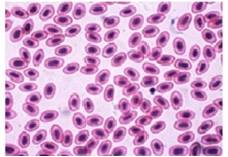
Fig. 7 Lymphocytes (Control)
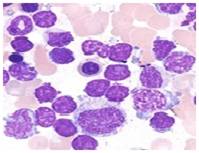
Fig. 8 Lymphocytes (Infected)
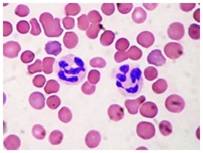
Fig. 9 Neutrophils (Control)
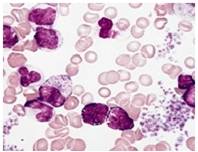
Fig. 10 Neutrophils (Infected)
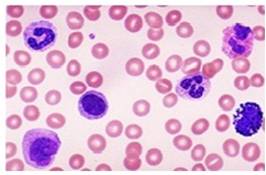
Fig. 11 Monocytes (Control)
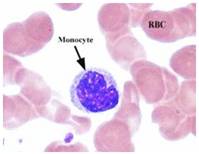
Fig. 12 Monocytes (Infected)
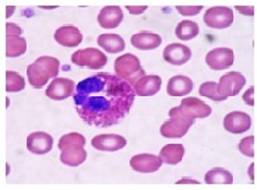
Fig. 13 Esinophils (Control)
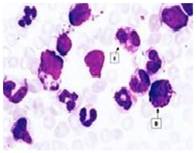
Fig. 14 Esinophils (Infected)
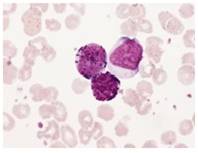
Fig. 15 Basophils (Control)
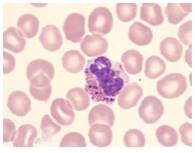
Fig. 16 Basophils (Infected)
ACKNOWLEDGEMENT- The authors would like to thank the Department of Zoology, Kakatiya University, Warangal, for providing laboratory facilities.
REFERENCES
- Arias, C. Welker, T.L. Shoemaker, C.A. Abernathy, J.W. Klesius, P.H. 2004. Genetic fingerprinting of Flavobacterium columnare isolates from cultured fish. J. Appl. Microbiol. 97, 421-428.
- Ueda, I.K. Egami, M.I. Sasso, W.S. Matushima, E.R. 1997. Estudos hematológicosem Oreochromi sniloticus (Linnaeus, 1758) (Cichlidae, Teleostei) – Parte I. Braz. J. Vet. Res. Anim. Sci. 35 (5), 270-275.
- Sebastião, F. A. D. Nomura, R. Sakabe, F. Pilarski. 2011. Hematology and productive performance of Nile tilapia (Oreochromis niloticus) naturally infected with Flavobacterium columnare. Brazilian Journal of Microbiology. 42: 282-289.
- Thompson K.D., Lilley J.H. and S. Chinabut. 1997. The antibody response of snakehead fish, Channastriata (Bloch), to Aphanomyces invaderis. Fish Shell fish Immunol. 7: 349-353.
- Chinabut S., Roberts R.J., Willoughby L.G. and M.D. Pearson. 1995. Histopathology of Snakehead, Channastriatus (Bloch), experimentally infected with the specific Aphanomyces fungus associated with Epizootic Ulcerative Syndrome (EUS) at different temperatures. J. Fish Dis., 18:41-47.
- Joseph S and Carnahan. 1994. The isolation, identification and systematic of the motile Aeromonas species. Annual Review of the fish Diseases 4:315-343.
- Azad IS, Rajendran KV, Rajan JJS, Vijayan KK and San-tiago TC. 2001. Virulence and histopathology of Aeromonas hydrophila (Sah, 93) in experimentally infected Tilapia Ore-ochromis mossambicus (L.). J. Aquac. Trop., 16, p. 265-275.
- Al-Harbi A and MN. Uddin. 2004. Seasonal variation in the intestinal bacterial flora of hybrid Tilapia (Oreochromis niloticus x Oreochromis aureus) cultured in earthen ponds in Saudi Arabia. Aquaculture, 229 (No. 1-4), 37-44.
- Cai WQ, Li SF and Ma JY. 2004. Diseases resistance of Nile Tilapia (Oreochromis niloticus), blue tilapia (Oreochromis aureus) and their hybrid (Female Nile tilapia x Male Blue tilapia) to Aeromonas sobria. Aquaculture, 229 (1-4), 79-87.
- Ramaswamy, M and Reddy, T.G. 1978. A comparative study of haematology of three air-breathing fishes. Proc. Indian Acad. Sci., 87B 12: 381-385.
- Rehulka J. 2002. Aeromonas causes severe skin lesions in rainbow trout (Oncorhynchus mykiss): clinical pathology, haematology and biochemistry. Acta Vet. Brno, 71, 3, p. 351-360.
- Martins ML, Tavares -Dias M, Fujimoto RY, Onaka EM and Nomura DT. 2004a. Haematological alterations of Leporinusmacro cephalus (Osteichthyes: Anostomidae) naturally infected by Goezialeporini (Nematoda: Anisakidae) in fish pond. Arq. Bras. Med. Vet. Zoot, 56, 5, p. 640-646.
- Anderson, DP. 2003. Disease of Fishes. Narendra Publishing House, Delhi pp. 22-73.
- Bruno, DW and Munro, ALS. 1986. Haematological assesement of rainbow trout Salmogairdneri. Richardson and Atlantic Salmon salar. L.., infected with Renibacterium salmoninarum. J. Fish Dis. 9, 194 – 204.
- Haniffa MA, Marimuthu K. Seed production and culture of snakehead. Infofish Int. 2004. 2:16-18.
- Hurbee TC and Smith SA. 2000. Haematology of fish. In Schalm’s Veterinary Haematology,5th edition Edited by Feldman , B,F .Zinki , J.G and Jain, N.C. Lippincott Williams and Wilkins (USA), 34 . 1120 –1125.
- Sahli’s. 1962. Detemination of haemoglobin by acid haematin method. In practical haematology (Ed: Dacie J. V. and Lewis S.M) 5th Edn.Churuchill, London.
- Moyle, P.B and Cech, J.J. 1982. An Introduction Ichthyology. First Edition, Prentis Hall, Inc. Eagle wood cliffs, New Jeresy, pp 396-409.
- Younus Ahmad, Syed Mudasir Andrabi1, Altaf Hussain, Gowher Hussain, Abid Rashid Wani, R.C Bannetwala and Mahesh Tharani. 2015. Haematological studies of fresh water fish, labeo rohita (ham.) Exposed to heavy metals. International Journal of Science, Environment and Technology, Vol. 4, No 6, 1513 – 1523.
- Mahajan, C.L and Dheer, J.M.S. 1979b. Cell types in the peripheral blood of an air-breathing fish, Channa punctatus. J. Fish Biol., 14:481-487.
- Devi, G., Baruah, B.K and Das, M. 2004. Study on the effect of paper mill effluent on Haematological profile of Heteropneustes fossilis (Bloch), Poll. Res. 23 (4): 611-614.
- Xavier Innocent B, M. Syed Ali Fathima and A. Sivagurunathan. 2011. Haematology of Cirrhinuus mrigala fed with Vitamin- C supplemented diet and post challenged by Aphanomyces invadans. Journal of Applied
- Pharmaceutical Science: 01: (09), 141-144.
- Malathi K, Kannathasan A and Rajendran K. 2012. Comparative Haematological Studies on Fresh Water Fishes Channapunctatus and Channastriatus (BLOCH). International journal of Pharmaceutical, Chemical and Biological Sciences. 2 (4), 644-648.
- Gautham, A.K and M.L. Gupta. 1989. Chromium induced haematological anomalies in a freshwater fish Channapunctatus (Bloch) J. Environ. Biol. 10(3): 239- 243.
- Laxmareddy B and Benarjee, G. 2013. Intestinal histopathology of trematode infected fish, Channa striatus. Biolife, 1: (1), 29-31.
- Faisal M. and W.J. Hargis. 1992. Augmentation of mitogen-induced lymphocyte proliferation in Atlantic menhaden, Brevoortia tyrannus, with ulcer disease syndrome.Fish Shellfish Immunol. 2:33-42.
- Mohan Goger and Vijay sawanth. 1989. Haematological changes during uranyl nitrate indiced acute toxicity. Effect of nitrate induced acute toxicity. Effect of sodium loading. J. Environ. Biol., 10 (1): 35-41.
- Thakur, G.K and Pandey, P.K. 1990. BHC (Gammaxene) poisoning effect on leucocytes of an air breathing fish. Clarias batrachus. (Linn). J. Env. Bio.11 (2): 105-110.
| Source of Financial Support: Nil Conflict of interest: Nil |
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |