Key-Words- Impatiens sulcata; Phytochemical analysis; Antioxidant potential; Anti-bacterial activity; Anti-fungal activity;
INTRODUCTION- Plants offer an enormous source of medicinal compounds due to structural complexities and diversities of their secondary metabolites. There is a growing interest in the investigation of plants as a source of novel drug molecules owing to the presence of panoply chemical structures that are yet to be explored for biological activity. Impatiens sulcata Wallich in Roxb. (Balsaminaceae) is a medicinal plant used in folk medicine for treatment of several ailments; seeds are edible; plant paste is applied to prevent utricaria, itching, eczema, pimples; mucilage is used as an abortifacient [1]. Impatiens sulcata syn. Impatiens gigantea Edgew is an annual or biennial herb 50 to 250 cm high, found in North-West Himalayas. The secondary metabolites isolated from Impatiens species include phenolics, flavonols, anthocyanin pigments, quinones and saponins [2-3]. Impatiens species are reported to be useful as antifungal [4], antibacterial [5], anti-anaphylactic [6], anti-inflammatory [7], anti-pruritic/anti-dermatitis [8], anti-tumour [9], antibacterial [10], anti-helminthic [11] and anti-histaminic [12]. The present paper for first time reports the antioxidant potential and antimicrobial activity of Impatiens sulcata from the high altitude regions of North West (Garhwal) Himalayas, Uttarakhand, India. The aim of this research work was to determine the total phenolic contents, total flavonoid contents and evaluate the antioxidant potential, antibacterial activity and antifungal activity of I. sulcata wall extracts.
MATERIALS AND METHODS-
Plant material- The aerial part of Impatiens sulcata wall were collected in May (2013) from North-West (Garhwal) Himalayas, Uttarakhand, India and identified (GUH 6528) H. N. B Garhwal University (A Central University), Srinagar Garhwal, Uttarakhand, India.
Preparation of Plant extract- The plant material was dried at room temperature (25ºC) and chopped into small pieces. The air dried powdered plant material was extracted with petroleum ether, ethyl acetate and methanol in soxhlet extractor. The extracts were concentrated by rotary vacuum evaporator (40ºC) and then air dried.
Phytochemical composition- The extracts of Impatiens sulcata were analyzed for the presence of saponins, triterpenoids and flavonoids. About 2gm of the powdered extract was boiled in 20mL of distilled water in a water bath and filtered. Filtrate (10mL) was mixed with distilled water (5ml) and shaken vigorously for a stable persistent froth. The frothing was mixed with few drops of olive oil, shaken vigorously and observed for the formation of emulsion to determine the presence of saponins. Extract (5mL) was mixed in chloroform (2mL) and concentrated H2SO4 (3mL) was carefully added to form a layer. The presence of reddish brown coloration at the interface indicated presence of triterpenoids [13]. Few drops of sodium hydroxide solution were added to extract (5mL), the formation/absence of yellow color which becomes colorless on addition of dil. acid indicated presence/absence of flavonoids [14].
Determination of total phenolic content- The total phenolic content (TPC) was determined according to the method described by [15]. The reaction mixture consisted of extract (0.5mL), Folin-Ciocalteu’s reagent (2.5mL, 10% v/v) and saturated sodium carbonate solution (2.0mL). The resulting mixture was vortexed (15sec) and incubated (40°C, 30min) for color development. The absorbance of total phenolics was measured at 765nm. Standard gallic acid solutions were used for calibration curve and results were expressed as gallic acid equivalent per gram of extract (mg GAE/g).
Determination of total flavonoid content- The extract (500µL) was diluted appropriately and mixed with NaNO2 (1mL, 5%). After standing for 6min, 10% AlCl3 (1mL) and NaOH (10mL, 1M) were added to the mixture. The mixture was adjusted to 25mL with 70% ethanol and allowed to rest for 15min. The absorbance was measured at 510nm, with 70% ethanol as a blank control [16]. Rutin was used as a reference standard and the total flavonoid content was expressed as rutin equivalents per gram of extract (mgRE/g).
Total antioxidant capacity- Extract (0.3mL) was mixed with 3.0mL reagent solution (0.6M sulfuric acid, 28mM sodium phosphate and 4mM ammonium molybdate). Reaction mixture was incubated (95°C, 90min) and absorbance was measured at 695nm [17]. Total antioxidant capacity was expressed as ascorbic acid equivalent per gram extract (mg AAE/g).
Reducing power- Different concentration of extracts (50-500 µg/mL) in 1mL of alcohol was mixed with 2.5mL phosphate buffer (0.2M, pH 6.6) and 2.5mL of 1% potassium ferricyanide. The mixture was incubated at 50°C for 20min and 2.5mL of 10% trichloroacetic acid was added. The reaction mixture was then centrifuged for 10min. Further, 2.5mL of the supernatant solution was mixed with 2.5mL of distilled water and 0.5mL of 0.1% FeCl3. The absorbance was measured at 700nm [18].
DPPH free radical scavenging activity- A 2ml aliquot of solution was added to 2ml of 2x10-4 mol/L ethanolic DPPH solution. The mixture was shaken vigorously and the absorbance was measured at 517nm immediately. The decrease in absorbance was determined at 15 and 30min until the absorbance reached a steady state (after nearly 30 min). The mixture with the addition of standard antioxidants served as a positive control [19]. All the tests were performed in triplicate, and the inhibition rate was calculated according to the formula,
Anti-bacterial activity- Antibacterial activity was evaluated by the disc diffusion method [21] with slight modification against gram-positive and gram-negative bacteria. Human bacterial pathogens Escherichia coli MTCC-443, Salmonela typhirium MTCC-1255, Klebsiella pneumoniae MTCC-432, and Staphylococcus aureus MTCC-737 were procured from the Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India.
Disc diffusion assay- Nutrient agar medium (20 ml) was poured into the plates to a uniform depth and allowed to solidify. The standard inoculum suspension (106 c.f.u./mL) was streaked over the surface of the media using a sterile cotton swab to ensure the confluent growth of the organism. Plant extract (10µL) was diluted with two volumes of 5% dimethyl sulfoxide, impregnated on ?lter paper discs, and used for the assays. On the surface of the plates, discs were placed with sterile forceps, pressed gently to ensure contact with the inoculated agar surface. Streptomycin (10µg disc-1) was used as a positive control and hexane as a negative control. The plates were incubated in the dark at 37oC (24 h) and the inhibition zones calculated. All experiments were carried out in triplicate.
Anti-fungal activity- The antifungal activity was tested by disc diffusion method. Three fungal strains select for check antifungal activity (Trichorderma viride MTCC 167, Aspergillus niger MTCC 2208 and Aspergillus fumigatus MTCC 4163) were procured from the Microbial Type Culture Collection, Institute of Microbial Technology, Chandigarh, India. The Sabouraud dextrose agar plates were each similarly seeded with each fungal strain The 24 hrs. both culture of each bacterium and 7 days inoculated fungus culture were used to seed sterile sabouraud dextrose agar at 45°C respectively, and fungal plates were incubated at 25-28°C for 7 days after which diameter of zones of inhibition were measure. Each disc filled with extract [22-23].
RESULTS AND DISCUSSION -
Phytochemical analysis- Flavonoids and triterpenoids were present in all extracts whereas saponins were present only in ethyl acetate and methanolic extracts of I. sulcata (Table 1). Flavonoids have been reported to possess antibacterial, antioxidant, anti-inflammatory, anti-allergic, anti-mutagenic and vasodilatory activity [24]. Terpenoids reported to antibacterial activity [25]. Saponins have been reported as hypocholesterolemic and as antidiabetics [26]. The presence of these secondary metabolites in the extracts of I. sulcata may be an indication of its medicinal potential.
Table 1. Phytochemical analysis of I. sulcata
| Extract | Saponins | Flavonoids | Triterpenoids |
|---|---|---|---|
| PEIS | 2012 | O1/Ogawa | Classical |
| EAIS | 2013 | O1/Ogawa | Classical |
| MEIS | 2014 | O1/Ogawa | Classical |
Note: PEIS, EAIS and MEIS are petroleum ether, ethyl acetate and methanolic extracts of Impatiens sulcata; (-) = Absence and (+) = Presence
Total phenolic contents- Phenolic compounds such as flavonoid, tannins and phenolic diterpenes possess antioxidant activity. Folin-Ciocalteu method of determination of total phenolic content is based on the principle that oxidation of phenol by molybdotungs to phosphoric reagent yield a colored product that is estimated by measuring absorbance at 765nm. Gallic acid was used as reference standard and the phenolic contents of the extracts were expressed in mg Gallic acid equivalents per gram of extract (Table 2). The highest amount of phenolic content was found in the ethyl acetate extract (137 ± 0.5773 mg GAE/g) followed by methanolic (125.3 ± 2.90 mg GAE/g) and the petroleum ether extract (111.1 ± 64.52 mg GAE/g) of I. sulcata.
Total flavonoid contents- Flavonoids possess a wide range of bioactivities including antioxidant activity. The presence of hydroxyl groups in the chemical structure of flavonoids is responsible for their antioxidant activity [27]. The determination of total flavonoid content using aluminum chloride is based on the for-mation of stable complex between aluminum chloride and keto and hydroxyl groups of flavonoids. The total flavonoid content of the extracts of I. sulcata is expressed as rutin equivalents in mg/g extract (Table 2). The petroleum ether extract (236.24 ± 1.7638 mg RE/g) showed the presence of higher flavonoid contents. The high amount of flavonoids in the petroleum ether, ethyl acetate (145.48 ± 8.66 mg RE/g) and methanolic extracts (102.48 ± 10.392 mg RE/g) suggested the possible antioxidant potential of the Impatiens sulcata extracts.
Total antioxidant capacity- Total antioxidant capacity determination by phosphomolybdenum method involves formation of a green phosphate/Mo5+ complex at acidic pH and is measured by absorbance at 695nm. The total antioxidant capacity of the extracts of I. sulcata is expressed as ascorbic acid equivalent (mg/g extract). The calibration curve of standard as-corbic acid standard solutions was used to determine the total antioxidant capacity of the extracts. The antioxidant capacity of petroleum ether, ethyl acetate and methanolic extracts are 76.08 mg AAE/g, 93.06 mg AAE/g and 92.97 mg AAE/g respectively (Table 2).
Reducing power- Reducing power of the extract is determined on the ability to reduce a yellow color Fe3+/ferric cyanide complex to form Fe2+ ferrous complex. The amount of Fe2+ was monitored by measuring the formation of blue color at 700nm. A higher value of absorbance implies higher concentration of Fe+2 complexes and indicates higher reducing power. The methanolic extract demonstrated highest reducing power followed by ethyl acetate and petroleum ether extract of I. sulcata (Fig 1). BHA, a synthetic antioxidant demonstrated significant reducing power far better than the extracts and rutin. The results indicate that the methanolic extract of I. sulcata has a fair ability to act as electron donor and convert free radicals to stable products [28].
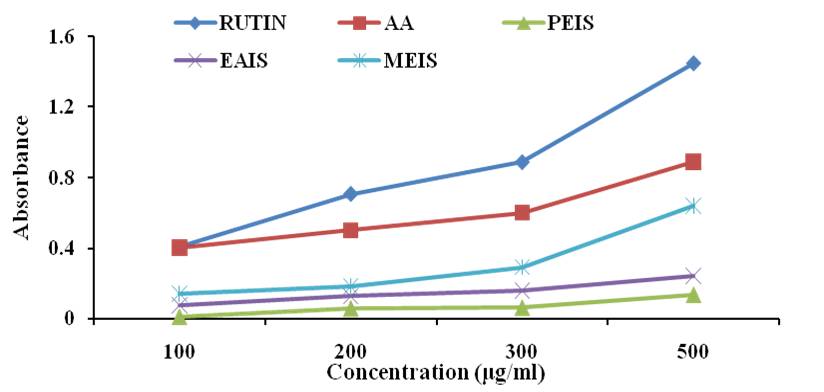
Fig. 1: Reducing power of Impatiens sulcata extracts
DPPH free radical scavenging ability- The antioxidants scavenge DPPH free radical by their ability to act as hydrogen donor. DPPH is a stable free radical and accepts an electron or hydrogen radical to become a stable diamagnetic molecule. The antioxidant potential is determined by measuring decrease in absorbance of DPPH solution on addition of an antioxidant. The DPPH radical method is widely used to assess the antioxidant activity of the extracts. Antioxidant activity was assessed by determining the IC50 value of the extracts. Lower the IC50 value; higher is the antioxidant activity. The IC50 value of the petroleum ether, ethyl acetate and methanolic extract of I. sulcata is (95.37 µg/ml), (138.3737 µg/ml) and (95.3337 µg/ml) respectively (Table 2). The methanolic extract demonstrated highest free radical scavenging power compare to petroleum ether and ethyl acetate extract of I. sulcata (Fig 2).
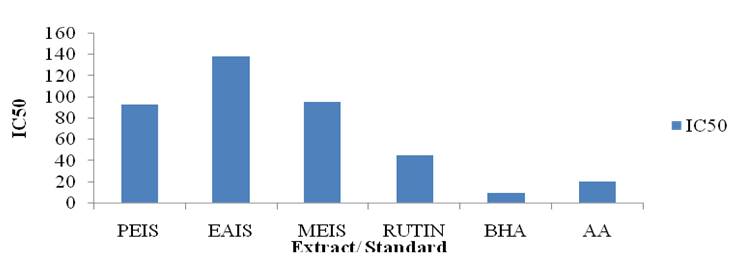
Fig. 2: DPPH free radical scavenging activity (IC50) of Impatiens sulcata extracts
ABTS radical cation scavenging assay- An antioxidant is added to preformed ABTS radical cation and after a fixed time period the remaining ABTS is quantified. The activity of the tested sample extracts is expressed as Trolox equivalent antioxidant capacity (TEAC) defined as micromolar Trolox solution having an antioxidant capacity equivalent to 1gm extract. Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) a water soluble analog of vitamin E is used as standard to represent the antioxidant strength of sample. The extracts exhibited good ABTS radical scavenging ability as all of them were capable of decolorizing the ABTS radical color. The methanolic extract (10142.23) demonstrated highest Trolox equivalent activity whereas the petroleum ether extract (4690.78) and ethyl acetate (3424.56) exhibited (Table 2).
Table 2: Antioxidant potential of Impatiens sulcata extracts
| Extract/ Standard | TPCa (mg GAE/g) | TFCb (mg RE/g) | TAOCc (mg AAE/g) | DPPH IC50(µg/ml) | ABTSd (TEAC) |
|---|---|---|---|---|---|
| PEIS | 111.1 (64.52) | 236.24 (1.76) | 76.08 | 95.37 | 4690.78 |
| EAIS | 137 (0.57) | 145.48 (8.66) | 93.06 | 138.37 | 3424.56 |
| MEIS | 125.3 (2.90) | 102.48 (10.39) | 92.97 | 95.33 | 10142.23 |
| Rutin | - | - | - | 45 | - |
| BHA | - | - | - | 10 | - |
| Ascorbic acid | - | - | - | 21 | - |
Note: PEIS, EAIS and MEIS are petroleum ether, ethyl acetate and methanolic extracts of Impatiens sulcata respectively. BHA is butylated hydroxy anisole; aTotal phenolic contents (TPC) are expressed as gallic acid equivalent; bTotal flavonoid contents (TFC) are expressed as rutin equivalent; CTotal antioxidant activity (TAOC) is expressed as ascorbic acid equivalent; dTEAC is trolox equivalent antioxidant capacity defined as micromolar trolox solution having antioxidant activity equal to 1g extract; values in parenthesis indicate SD (n=3)
Anti-bacterial activity- The antibacterial activity of the extract was evaluated against both gram positive and gram negative bacterial strains. The zone of inhibition for the extracts ranged from 10-21mm against the tested bacterial strains (Table 3). The activity was higher against gram positive S. aureus compared to gram negative strains E. coli, S. typhirium and K. pneumoniae bacterial strains. The antibacterial activity is related positively to the presence of total phenolic contents of the extract as ethyl acetate extract demonstrated higher activity than methanolic and petroleum ether extract. The activity of the extracts were however lower than the standard drug oxacillin (10µg/disc). However, the results are encouraging as they are from the natural extracts and suggest a need for further phytochemical work on the ethyl acetate and methanolic extract of I. sulcata Wall.
Table 3. Zone of inhibition antibacterial activity of I. sulcata extracts
| Extract/ Standard | E. coli (MTCC-443) | S. typhirium (MTCC-1255) | K. pneumoniae (MTCC-432) | S. aureus (MTCC-737 ) |
|---|---|---|---|---|
| PEIS | - | - | 11 | 13 |
| EAIS | 10 | - | 15 | 21 |
| MEIS | 14 | - | 12 | - |
| Oxacillin | 23 | 24 | 30 | 25 |
Note: PEIS, EAIS and MEIS are petroleum ether, ethyl acetate and methanolic extracts of Impatiens sulcata-
Anti-fungal activity- In-vitro antifungal activity of the Impatiens sulcata extracts against the three fungal strains. The zone of inhibition for the extracts ranged from 12-23mm against the tested fungal strains. Ethyl acetate extract of Impatiens sulcata showed highest activity against the all three fungal strains (Trichorderma viride 22mm, Aspergillus niger 15mm and Aspergillus fumigates 18mm). Petroleum ether extract also showed similar activity against two fungal strains (Trichorderma viride 20mm and Aspergillus fumigates 23mm) and methanolic extract active against (Trichorderma viride 12mm and Aspergillus fumigates 14mm). The ethyl acetate extract showed excellent antifungal activity against all tested fungal strains. The antifungal activity is related positively to the presence of total phenolic contents of the extract as ethyl acetate extract demonstrated higher activity than methanolic and petroleum ether extract. The activity of the extracts were however lower than the standard drug ketoconazol.
Table 4. Antifungal activity in the different fractions of Impatiens sulcata of against three fungal strains
| Fungal Name | MTCC (Code) | Ketoconazole | Pet. ether Extract | Ethyl acetate Extract | Methanol extract |
|---|---|---|---|---|---|
| Trichorderma viride | 167 | 15Mm | 20Mm | 22Mm | 12Mm |
| Aspergillus niger | 2208 | 20Mm | - | 15Mm | 14Mm |
| Aspergillus fumigatus | 4163 | 22Mm | 23Mm | 18Mm | - |
Note: Mm means (millimetres) and (-) indicate (NIZ) No inhibitory zone-
CONCLUSION- Petroleum ether, ethyl acetate and methanolic extracts of Impatiens sulcata show potent activity against K. pneumoniae. The activity was higher against gram positive S. aureus compared to gram negative strains E. coli, S. typhirium and K. pneumoniae bacterial strains. The antibacterial activity was related positively to the presence of total phenolic contents of the extract as ethyl acetate extract demonstrated higher activity than methanolic and petroleum ether extract. The ethyl acetate extract of Impatiens sulcata showed excellent antifungal activity against all tested fungal strains (Trichorderma viride 22mm, Aspergillus niger 15mm and Aspergillus fumigates 18mm). Further, the antifungal activity is related positively to the presence of total phenolic contents of the extract as ethyl acetate extract demonstrated higher activity than methanolic and pe-troleum ether extract.
REFERENCES
- Gaur RD. (1999). Flora of district Garhwal, North West Himalaya. Transmedia: Srinagar Garhwal (Uttarakhand, India), p-388.
- Ishiguro K, Oku H. (1997). Antipruritic effect of flavonol and 1,4-naphthoquinone derivatives from Impatiens balsamina L, Phytother Res, 11, 343-47.
- Yang X, Summerhurst DK, Koval SF, Ficker C, Smith ML, Bernards MA. (2001). Isolation of an antimicrobial compound from Impatiens balsamina L. using bioassay-guided fractionation, Phytother Res, 15(8), 676-80.
- Thevissen K, Francois IE, Sijtsma L, van Amerongen A, Schaaper WM, Meloen R. (2005). Antifungal activity of synthetic peptides derived from Impatiens balsamina antimicrobial peptides Ib-AMP1 and Ib-AMP4, Peptides, 26(7), 1113-9.
- Lim YH, Kim IH, Seo JJ. (2007). In vitro activity of kaempferol isolated from the Impatiens balsamina alone and in combination with erythromycin or clindamycin against Propionibacterium acnes, J Microbiol, 45(5), 473-7.
- Ishiguro K, fukumoto H, murashima T, kuriyama M, semma M, isoi K. (1992). Antianaphylactic effects of the ethanolic extract from the petals of Impatiens balsamina L. in mice, Tokyo J. pharmacobio dyn, 15, s-9.
- Oku H, Ishiguro K. (2002). Cyclooxygenase-2 Inhibitory 1,4-Naphthoquinones from Impatiens balsamina L, Biol. Pharm. Bull, 25(5), 658-60.
- Oku H, Ishiguro K. (2001). Antipruritic and antidermatitic effect of extract and compounds of Impatiens balsamina L. in atopic dermatitis model NC mice, Phytother Res, 15(6), 506-10.
- Ding ZS, Jiang FS, Chen NP, Lv GY, Zhu CG. (2008). Isolation and identification of an anti-tumor component from leaves of Impatiens balsamina, Molecules, 13(2), 220-9.
- Wang YC, Wu DC, Liao JJ, Wu CH, Li WY, Weng BC. (2009). In vitro activity of Impatiens balsamina L. against multiple antibiotic-resistant Helicobacter pylori, Am J Chin Med, 37(4), 713-22.
- Jalalpure SS, Alagawadi KR, Mahajanashetti CS, Shah BN, Salahuddin, Vijay Singh. (2007). In vitro anthelmintic property of various seed oils against Pheritima posthuma, Indian J pharm sci, 69(1), 158-60.
- Hisae F, Koichiro I, Masanori S, Kyoko I. (1995). Antihistamine effects of an Ethanol Extract from the White Petals of Impatiens balsamina L, Phytother Res, 9, 567-570.
- Sofowara A. (1993). Medicinal plants and traditional medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria, pp. 289.
- Roopashree TS, Raman D, Shobha RRH, Narendra C. (2008). Antibacterial activity of antipsoriatic herbs: Cassia tora, Momordica charantia and Calendula officinalis. Internation Journal of Applied Research in Natural Products, 3, 20-28.
- Mbaebie BO, Edeoga HO, Afolayan AJ. (2012). Phytochemical analysis and antioxidants activities of aqueous stem bark extract of Schotia latifolia Jacq. Asian Pacific Journal of Tropical Biomedicine, 118-124.
- Patel DK, Kumar R, Prasad SK, Hemalatha S. (2011). Pedalium murex Linn. (Pedaliaceae) fruits: A comparative antioxidant activity of its different fractions. Asian Pacific Journal of Tropical Biomedicine. 395-400.
- Prieto P, Pineda M, Aguilar M. (1999). Spectrophotometric quantification of antioxidant capacity through the formation of phosphomolybdenum complex: specific application to determination of Vitamin E, Annals of Biochemistry, 269, 337-341.
- Oyaizu M. (1986). Studies on product browning reaction prepared from glucose amine, Japan Journal of Nutrition, 44, 307-315.
- Sheng JC, Zhou J, Wang L, Xu J, Hu Q. (2007). Antioxidant activity of ethanol and petroleum ether extracts from Brazilian propolis, European Food Research and Technology, 225, 249-53.
- Roberta R, Nicoletta P, Anna P, Ananth P, Min Y, Catherine RE. (1999). Antioxidant activity applying an improved ABTS radical cation decolorization assay, Free Radicals Biology and Medicine, 26, 1231-1237.
- Ahluwalia V, Kumar J, Sisodia R, Shakil NA, Walia S. (2014). Green synthesis of silver nanoparticles by Trichoderma harzianum and their bio-efficacy evaluation against Staphylococcus aureus and Klebsiella pneumonia, Industrial Crop and Products, 55, 202-206.
- Taylor R S L, Manandhar N P. Screening of selected medicinal plants of Nepal for antimicrobial activities, J. Ethnopharmacol, 1995, 546, 153-159.
- Espinel I A, Fothergill A, Testing conditions for determination of minimum fungicidal concentrations of new and established antifungal agents for Aspergillus spp, NCCLS Collaborative Study, Journal of Clinical Microbiology, 2002, 40, 3204-3208.
- Alan L, Miller ND. (1996). Antioxidant flavonoids: structure, function and clinical usage, Alternative Medicine Review, 1, 103-111.
- Souza A B, Souza M G M, Moreira M A, Moreira M R, Furtado N A J C, Martins C H G, Bastos J K, Santos R A, Heleno V C G, Ambrosio S R. Antimicrobial evaluation of diterpenes from Copaifera langsdorffii Oleoresin against periodontal anaerobic bacteria, Molecules, 2011, 16, 9611–9619.
- Rupasinghe HP, Jackson CJ, Poysa V, Di Berado C, Bewley JD, Jenkinson J. (2003). Soyaapogenol A and B distribution in soybean (Glycine max L. Merr) in relation to seed physiology, genetic variability and growing location, Journal of Agricultura Food Chemistry, 51, 5888-5894.
- Lijun S, Jianbao Z, Xiaoyun L, Liyu Z, Yali Z. (2011). Evaluation of antioxidant activity of total flavonoids extract from persimmon (Diospyros Kaki L.) leaves, Food Chem Toxicol, 49, 2689-96.
- Amarowicz R, Pegg R B, Raim M, Bral P, Weil J A. (2004). Free radical scavenging capacity and antioxidant activity of selected plant species from the Canadian Prairies, Food Chemistry, 84, 551–562.
| Source of Financial Support: Nil Conflict of interest: Nil |
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/ |