Key-Words- Medicinal plants, Antimicrobial activity, Antifungal activity
INTRODUCTION- The use of natural products with therapeutic properties has a long history whereas plant, animal, and mineral products were the main source of medicines [1]. Many efforts have been made to discover new antimicrobial compounds from various kinds of sources such as mi-cro-organisms, animals and plants. Systematic screening of them may result in the discovery of novel effective antimicrobial compounds [2]. Plants can possess antimicrobial natural products to protect themselves from microbial infection and deterioration [3].
In the developing countries, synthetic drugs are not only expensive and inadequate for the treatment of diseases but are also often with adulterations and side effects [4]. In recent years, concern over pathogenic and spoilage microorganisms in foods has increased due to the increase in outbreaks of food borne disease [5]. There are growing interests in using natural antimicrobial compounds, es-pecially extracted from plants, for the preservation of foods. In addition, there are more consumers who tend to question the safety of synthetic additives and would prefer natural foodstuffs [6-7]. There is therefore the need to search for plants of medicinal value. The plant used in the present study was Ocimum santum (Tulsi), Withnia somnifera (Ashwgandha) Santalum paniculatumi (Chandan), Aloe-vera, shatavari. [8]The extract from the leaves of these plants used in Malaria, Bronchitis, Gastric disorders, Cough, cold etc. In recent years more attention has been given to no chemical systems for seed treatment to protect them against seed-borne pathogens. Plant extracts have played significant role in the inhibition of seed-borne pathogens and in the improvement of seed quality and field emergence of plant seeds.
[9] Below is a list of some of the nastiest skin diseases which are fatal eczema, psoriasis, plaques and rashes on skin, scleroderma, and herpes gladiatorum. Wound healing is an important biological process involving tissue repair and regeneration. The skin disease curing activities of plants have since been explored. The significant successes recorded have led to investigation into medicinal plants with a view to confirming these acclaimed properties. In this study we record the different parts of plants of India used for curing skin disease containing some active principles or components that are antimicrobial in function. In this way we have made an attempt to give an insight into the different parts of herbs having po-tential use in different skin disease, which could be beneficial in therapeutic practice.
MATERIALS AND METHODS
PLANTS USED- Different plants and their extract where collected to check their antimicrobial activity against different mi-croorganism. In this study 19 plants where used. Leaf extract of different plant where collected from different region of Latur and live plants were also maintained in pots.
Table 1: Information about different medicinal plants
| S. No. | Common name | Family | Botanical name | Plant part used |
|---|---|---|---|---|
| 1. | Tulsi | Labiatae | Ocimum sanctum | Leaves, stem |
| 2. | Neem | Meliaceae | Azadiracta indica | Leaves, stem |
| 3. | Adulsa | Acanthaceae | Adhatida vasica | Leaves |
| 4. | Gudmar | Asclepiadaceae | Gymnema sylvestre | Leaves |
| 8. | Chandan | Santalaceae | Santalum paniculatum | Leaves |
| 9. | Jakhamjodi | Asteraceae | Tridax procumbens | Leaves |
| 10. | Shatavari | Asparagus racemosus | Leaves | |
| 11. | Akarkara | Anacyclus pyrethrum | Leaves | |
| 12. | Ashwagandha | Solanaceae | Withania somnifera | Leaves |
| 14. | Karanj | Pongamia pinnata | Leaves | |
| 15. | Nivdung | Cactaceae | Opuntia | Leaves |
| 16. | Prickly pears | Cactaceae | Opuntia | Leaves, fruits |
| 17. | Ashoka | Sillago indica | Saraca indica | Bark, flowers |
| 18. | Unknown 1 | Leaves | ||
| 19. | Unknown 2 | Leaves |
Preparation of Plant Extracts- The plant materials (leaves) were washed thoroughly with distilled water and alcohol and again washed with sterile distilled water. Plant leaves homogenized (grind) by using mortal and pestal by adding distilled water. 10 ml water was used for 1gm plant materials. Extract filtered by using muslin cloth and used for experiment.

Fig: 1 Extraction sample of different plants
Test organism used: E. coli, Salmonella typhi, Staphylococcus aureus, Proteus vulgarisi
Fungus used: Candida albicans, Aspergillus fotidus, Aspergillus niger, Penicillum chrysosoprium
EXPERIMENTAL PROCEDURE
Well Diffusion method for Antibacterial Activity- This method depend on the diffusion of various extract from a well through a solidified agar layer Petri dish, so that the growth of inoculated microorganism is pre-vented entirely in circular zone around the prepared well containing plant extract using micropipette.
Prepared nutrient agar plates and spreaded the bacterial culture (E. coli, Staphylococcus aureus, Salmonella typhi, Proteus vulgarisi) on agar surface medium. Made wells on the surface of agar (6mm) with help of cork borer and added 50”l of plant extract in well. For the diffusion of plant extract in the agar, kept plates in refrigerator for 15min. After the diffusion plates were incubated at 37șC for 24hr in incubator. Observed the zone of inhibition after incubation period.
Disc Method: Prepared nutrient agar plates and spreaded the bacterial culture (E. coli, Staphyloccocus aureus, Salmonella typhi, and Proteus vulgarisi) on agar surface medium. Made a circular disc (5mm in diameter) deep in the plant extract, put this disc on agar surface.
For the diffusion of plant extract in the agar, keep plates in refrigerator for 15min. After the diffusion plates are incubated at 37șC for 24hr in incubator. Observed zone of inhibition after incubation period.
Agar disk diffusion assay: The Agar disk diffusion method of antimicrobial test was developed in 1940 [10]. The procedure which was accepted by NCCLS and widely used now a days, is a modification of that described by Bauer, Kirby, Sherris and Truck (commonly known as Kirby-Bauer test) [11-12]. The Agar disk diffusion technique has been widely used to assay plant extract for antimicrobial activity [13-15]. In this method, 6 mm sterilized filter papers disks (Whatmann No. 1) are saturated with filter sterilized [16] plant extract of desired concentration.
The impregnated discs are then placed onto the surface of a suitable solid agar medium like Mueller Hinton, Trypton soya agar [17] or Nutrient agar [18].
The media has been pre-inoculated with test organisms. The standard inoculum size is of 1 x 108 CFU/ml of bacteria for inoculating diffusion plates [19] which is equal to McFarland 0.5 turbidity standard. Some researchers impregnate the paper disk with plant extract before putting on the inoculated plates while others prefer after [19].
The drying time of impregnated paper disk varies among researchers from 2 h to overnight under a laminar flow cabinet.
Well Diffusion Method for Antifungal Activity- This method depends on the diffusion of various ex-tract from a well through a solidified agar layer Petri dish, so that the growth of inoculated fungus is pre-vented entirely in circular zone around the prepared well containing plant extract sing micropipette. Pre-pared czpadox agar plates and spreaded the fungal culture (Aspergillus niger, Candida albicans, Penicillum chrysosporium, Aspergillus fotidus) on the agar surface medium. Made wells on the surface of agar (6 mm) with help of cork borer. And add a 50”l of plant extract in well. For the diffusion of plant extract in the agar, keep plates in refrigerator for 15min. After the diffusion plates are incubated at 27șC for 24hr in incubator. Observed the zone of inhibition after incuba-tion period.
Disc Method: Prepared czpadox agar plates and spread the fungal culture (Aspergillus niger, Candida albicans, Penicillum chrysosporium, Aspergillus fotidus) on agar surface medium. Made a circular disc (5mm in diameter) and deep in the plant extract, put these discs on agar surface. For the diffusion of plant extract in the agar, keep plates in refrigerator for 15min. after the diffusion plates are incubated at 37șC for 24hr in incubator. Observed zone of inhibition after incubation period.
Agar disk diffusion assay: The Agar disk diffusion method of antimicrobial test was developed in 1940 [10]. The procedure which was accepted by NCCLS and widely used now a days, is a modification of that described by Bauer, Kirby, Sherris and Truck (commonly known as Kirby-Bauer test) [11-12]. The Agar disk diffusion technique has been widely used to assay plant extract for antifungal activity [20]. In this method, 6 mm sterilized filter papers disks (Whatmann No. 1) are saturated with filter sterilized [21] plant extract of desired concentration.
The impregnated discs are then placed onto the surface of a suitable solid agar medium like Mueller Hinton [20], czpadox agar [21]. The media has been pre-inoculated with test organisms. The standard inoculum size is of 1 x 108 CFU/ml of fungus for inoculating diffusion plates which is equal to McFarland 0.5 turbidity standard. Some researchers impregnate the paper disk with plant extract before putting on the inoculated plates [21]. While to drying time of impregnated paper disk varies among researchers from 2 h to overnight under a laminar flow cabinet [22]. Plates are then incubated for 48 h at 25°C (fungi) [23]. After incubation, zone diameter is measured to the nearest whole millimeter at the point wherein there prominent reduction of 80% growth.
RESULTS AND DISCUSSION- Properties of the plant used in the study are discussed in the introduction. Different plants parts are used in this study. The amount of residue extracted with water is high. The three bacterial species are used for the study E. coli, S. aureus, S. typhi, and fungal species are used Candida albicans, and Aspergillus niger.Ocimum sanctum has more zone of inhibition against C. albicans than others and the less zone of inhibition jakamjudi against A. niger.
Table 2: Shows the antimicrobial and antifungal activity of plant extracts (Where zone of inhibition measured in mm)
| S. No. | Name of plants | E. coli | S. aureus | P. vulgarisi | C. albicans | A. niger |
|---|---|---|---|---|---|---|
| 1. | Ocimum sanctum | 4 5 6 ±0.5, ±0.5, ±0.1 | 4 4.5 6 ±0.4,±0.5,±0.5 | - | 6 7 6 ±0.5, ±0.5, ±0.1 | - |
| 2. | Azadiracta indica | 3.5 4 5 ±0.5,±0.5,±0.1 | 4.5 5 5 ±0.5,±0.5,±0.2 | - | 4 4.5 4 ±0.3,±0.5,±0.5 | 3 4 4.5 ±0.5,±0.5,±0.1 |
| 3. | Gymnema sylvestre | - | 4.5 5 6 ±0.5,±0.4,±0.1 | - | 4 4.5 6 ±0.4,±0.5,±0.5 | - |
| 4. | Adhatoda vasica | - | 6 7 8 ±0.5, ±0.4, ±0.5 | - | 5 6 6.5 ±0.5,±0.4,±0.5 | - |
| 5. | Anacyclus pyrethrum | - | - | - | 6.5 7 8 ±0.5,±0.4,±0.5 | - |
| 6. | opuntia | - | - | - | - | 7.3 7.5 8 ±0.5,±0.4,±0.5 |
| 7. | Pongamia pinnata | - | - | - | - | - |
| 8. | Santalum paniculatum | - | 6.5 7 8 ±0.5,±0.4,±0.5 | - | - | - |
| 9. | Jakhamjodi | - | - | - | - | 3.5 4 5 ±0.5,±0.5,±0.1 |
| 10. | Withania somnifera | - | - | - | - | - |
| 11. | Berberis aristata | - | - | - | - | - |
| 12. | Aloevera barbadensis | - | - | - | - | - |
| 13. | Nivdung | - | - | - | - | - |
| 14. | Saraca indica | - | - | - | - | - |
| 15. | Unknown 1 | - | - | - | - | - |
| 16. | Unknown 2 | - | - | - | - | - |
| 17. | Mixed culture | 10 11 10 ±0.5,±0.5,±0.5 | 11 11 12 ±0.4,±0.5,±0.5 | 6.5 7 7.5 ±0.1,±0.5,±0.5 | 10 11 12 ±0.5,±0.5,±0.5 | 9 10 11 ±0.5,±0.4,±0.5 |
| 18. | Control | 11.5 11 11 ±0.5,±0.5,±0.4 | 12 13 13 ±0.5,±0.5,±0.5 | 8 8 9 ±0.3,±0.5,±0.5 | 12 13 12 ±0.5,±0.5,±0.5 | 11 11 11 ±0.4,±0.5,±0.5 |

Fig: 2 Zone of inhibition Ocimum sanctum against C. albicans

Fig: 3 Zone of inhibition of Azadiracta indica against S. aureus

Fig: 4 Zone of inhibition of Adhatoda vasica against S. aureus

Fig: 5 Zone of inhibition of Gymnema sylvestre against E. coli
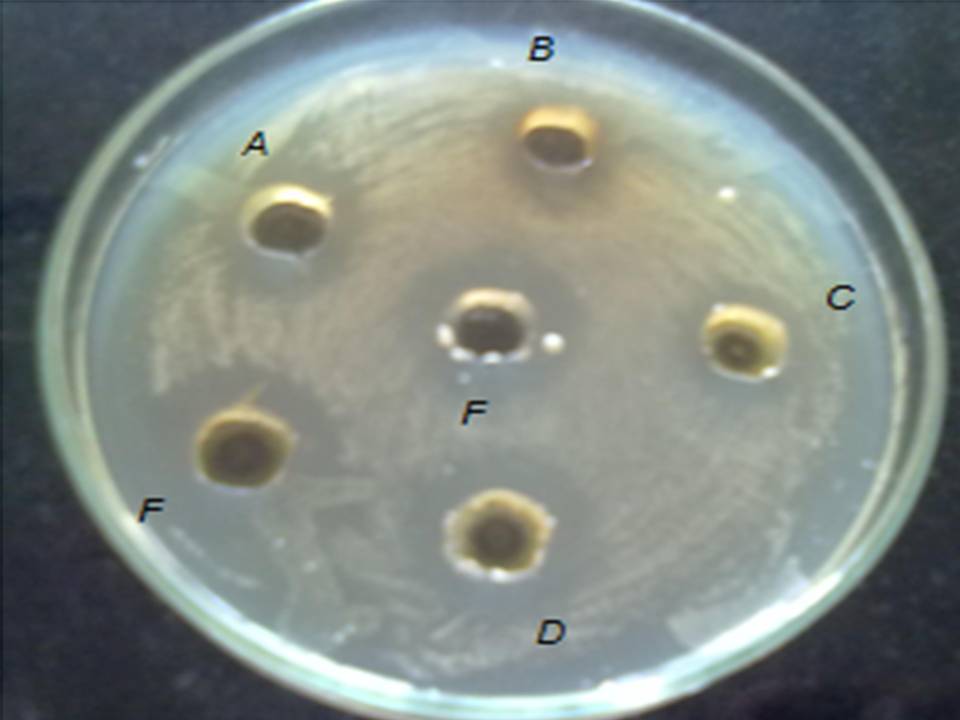
Fig: 6 Zone of inhibition of (A) Ocimum sanctum, (B) Azadirachta indica, (C) Alove vera barbadenesis , (D) Gymnema sylvestre, (E) Santalum album, (F) Anacyclus pyrethrum against S. aureus
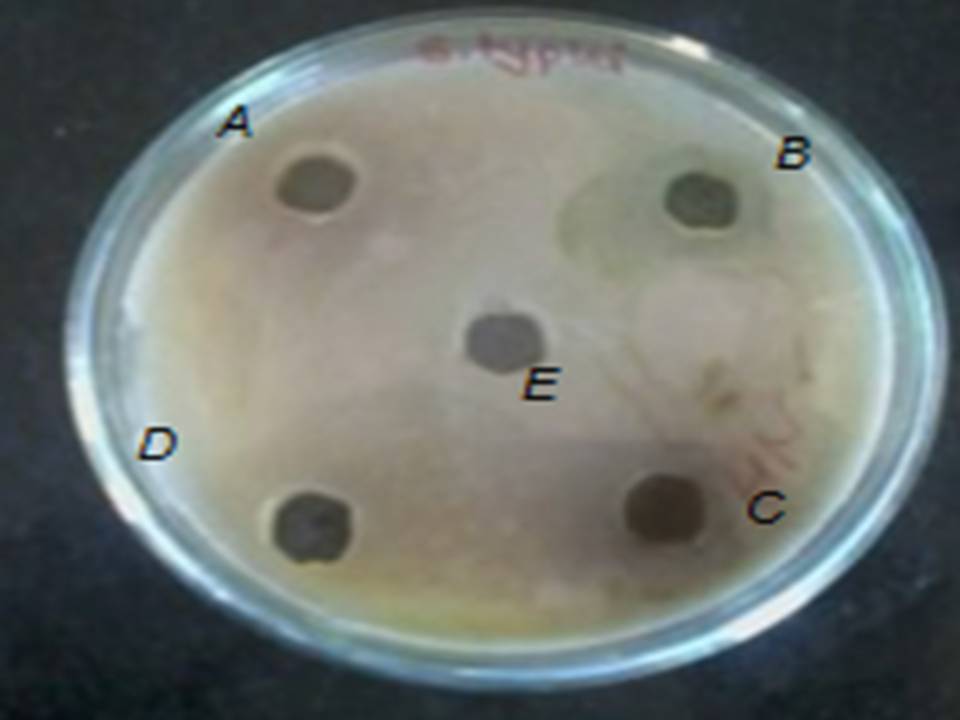
Fig: 7 Zone of inhibition of (A) Ocimum sanctum, (B) Azadirachta indica, (C) Alove vera barbadenesis, (D) Gymnema sylvestre, (E) Santalum album, (F) Anacyclus pyrethrum against S. typhi
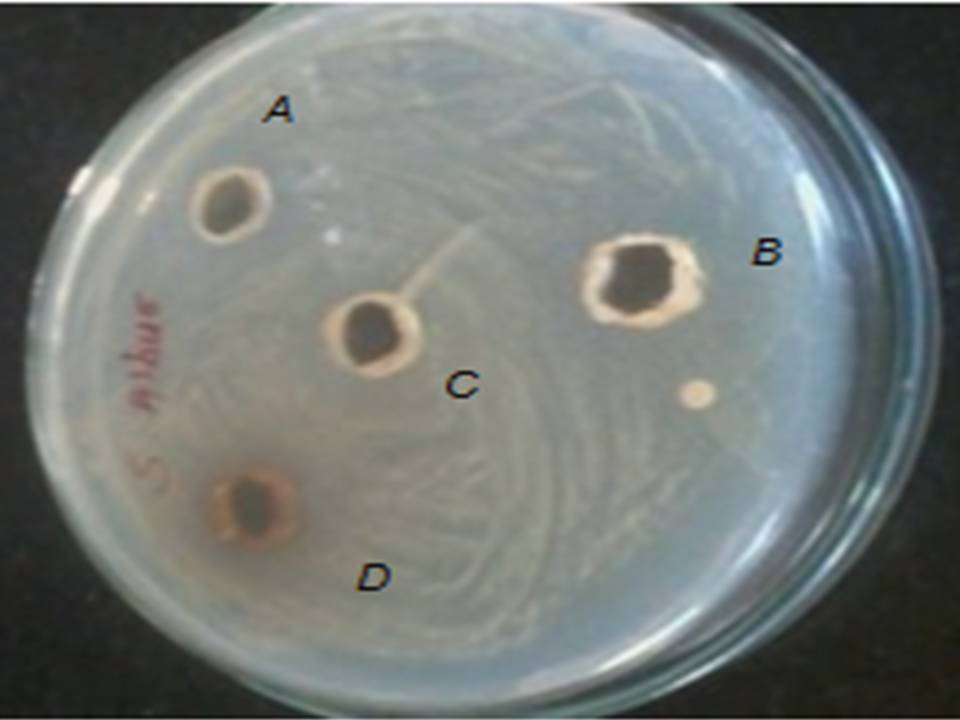
Fig.8. Zone of inhibition of (A) Santalum album, (B) Alove vera barbadenesis , (C) Azadirachta indica (D) Ocimum sanctum against S. aureus

Fig 9: Zone of inhibition of Ocimum sanctum against S. typhi
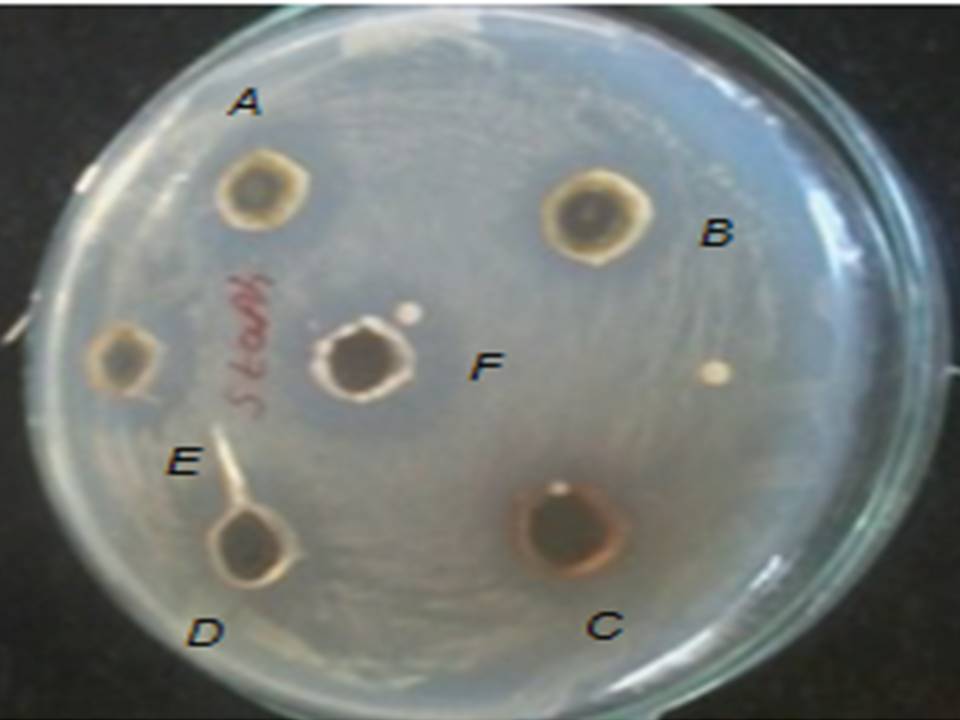
Fig: 10 Zone of inhibition of (A) Ocimum sanctum, (B) Azadirachta indica, (C)Alove vera barbadenesis , (D) Gymnema sylvestre, (E) Santalum album, (F)Anacyclus pyrethrum against S. aureus
- Abey singhe PD, Wijesekara D, Pathirana RN. 2003. Inhibition of growth of antibiotic resistant Staphylococcus sp. And Proteus sp. By Mangrove plant extracts. Proceeding of the First Science Symposium, University of Ruhana, pp: 1-9.
- Abeysinghe PD, Withanasam M, Pathirana RN, Abeysinghe S. 2002. Preliminary in vitro screening of antibacterial compounds of some mangrove plant extracts for clinical isolates from different sources. Proceeding of the First Science Symposium, University of Ruhana, pp: 22-25.
- Ali-Shtayeh, M.S., Abu Ghdeib, S.I 2000. Antimycotic activity of twenty-two plants used in folkloric medicine in the Palestinian area for the treatment of skin diseases suggestive of dermatophyte infection. Mycoses., 2000.
- Cowan, M.M., 1999. Plant Products as Antimicrobial Agents. Clinical Microbiology Rev., 12: 564-582.
- Ellof, J.N., 1998 Which extractant should be used for the screening and isolation of antimicrobial components from plants? Journal of Ethnopharmacology, 60: 1-8.
- Ikram M and Haq I 1984. Screening of medicinal plants for antimicrobial activity, Fitoterpia, part III, 55(1):62-64.
- Rauha, J.P., Remes, S., Heinonen, M., Hopia, A., Kahkonen, M., Kujala, T., Pihlaja, K., Vuorella, H., Vuorella, P (2000). Antimicrobial effects of Finnish plant extracts containing flavonoids and other phenolic compounds. Int. J. Microbiol., 56, 3-12.
- Taber, C. W. (1965) Tabers Cyclopedic Medical Dictionary, 10th edition, F. A. Davies Company, U. S.
- Thomas, J. C. (1997) Veterinary Pathology, 6th edition, Williams and Wilkin, Maryland, USA.
- Heatley, N.G. (1944). Method for the assay of agar disc diffusion. Biochemical Journal 38, 61-5.
- Bauer AW, Perry DM, Kirby WMM (1959). Single disc antibiotic sensitivity testing of Staphylococci. Arch. Int. Med. 104: 208-216.
- Bauer AW, Kirby WMM, Sherries JC, Turckp M (1966). Antibiotic susceptibility testing by a standardized single disk method. Am. J. Clin. Pathol. 45: 493-496.
- Freixa B, Vila R, Vargas L, Lozano N, Adzet T, Caniguera S (1996). Screening for antifungal activity of nineteen Latin American plants. Phytother. Res. 12(6): 427-430
- Salie F, Eagles PFK, Lens HMJ (1996). Preliminary antimicrobial screening of four South African Asteraceae species. J. Ethnopharmacol. 52(1): 27-33.
- Ergene A, Guler P, Tan S, Mirici S, Hamzaoglu E, Duran (2006). Antimicrobial and antifungal activity of Heracleum sphondylium subsp. artivinense. Afr. J. Biotechnol. 5(11): 1087-1089
- Salie F, Eagles PFK, Lens HMJ (1996). Preliminary antimicrobial screening of four South African Asteraceae species. J. Ethnopharmacol. 52(1): 27-33.
- Lourens ACU, Reddy D, Baser KHC, Viljoen AM, Van Vuuren SF (2004). In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J. Ethnopharmacol. 9: 253-258.
- Doughari JH (2006). Antimicrobial activity of Tamarindus indica Linn. Trop. J. Pharm. Res. 5(2): 597-603.
- Baris O, Gulluce M, Sahin F, Ozer H, Kilic H, Ozkan H, Sokmen M, Ozbek T (2006). Biological activities of the essential oil and methanol extract of Achillea Biebersteinii Afan. (Asteraceae). Turk. J. Biol. 30: 65-73.
- Mueller, Hinton (1941). A protein-free medium for primary isolation of the gonococcus and meningococcus. Proc. Soc. Exp. Biol. Med. 48: 330.
- Lourens ACU, Salie F et al). In vitro biological activity and essential oil composition of four indigenous South African Helichrysum species. J. Ethnopharmacol. 9: 253-258.
- Basri DF, Fan SH (2005). The potential of aqueous and acetone extracts of galls of Quercus infectoria as antibacterial agents. Indian J. Pharmacol. 37(1): 26-29.
- Baris O, Gulluce M, Sahin F, Ozer H, Kilic H, Ozkan H, Sokmen M, Ozbek T (2006). Biological activities of the essential oil and methanol extract of Achillea Biebersteinii Afan. (Asteraceae). Turk. J. Biol. 30: 65-73.
| Source of Financial Support: Nil Conflict of interest: Nil |
|---|
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/ |
|---|