Key-Words- Araceae, Arisaema, Asialofetuin, Antiproliferative effect, Apoptosis, Cytotoxicity Lectins, Mechanistic
INTRODUCTION- Colon cancer refers to cancerous growth in colon, rec-tum or caecum. It is most common malignancy worldwide and causes large scale morbidity and mortality [1]. Colon cancer is fourth most common cancer globally with 639,000 deaths reported annually [2]. It occurs most frequently in North America, Australia, New Zealand, Japan, India and Western Europe [3-5]. There are several risk factors related to colon cancer include presence of adenomatous polyps, previous history of ovary, uterus or breast cancer, contraction of specific strains of human papilloma viral infection, Streptococcus bovis and inflammatory bowel disease particularly ulcerative colitis.
In addition to that smoking, alcohol drinking, gender, ethnicity with higher risk in males than females and black than white, respectively [6-7]. As we know cancer develops due to uncontrolled division of altered cells in the body.
The escapes of regulation of these cells are, in most cases, caused due to aberrant glycosylation. The portion of these alternatively glycosylated molecules reach the blood stream. So they could be serve as early marker to enable cancer detection [8-9]. There is difference in glycosylation between malignant and healthy tissue which create excellent opportunities to identify sensitive and specific biomarker. Lectins are the natural biomolecules, which interact specifically with carbohydrates. “Lectins are protein of non immune origin that either bind to carbohydrate or sugar containing substance in a specific and reversible manner or precipitate glycoconjugates” [10]. These biomolecules can be used to differentiate malignanat stage from benign stage and benign stage from normal by studying the drgree of glycosylation in all the stages. Because the altered glycosylation induces either over ex-pression or under expression of some glycoproteins and expression of new sachharides. The lectin molecule known to mark the expression pattern with very high specificity, so that altered changes can be recognized. Lectins are majorly known to have inhibitory and cytotoxic effect on tumour cells by inducing programmed cell death (PCD) or apoptosis. Programmed cell death is highly regulated process that involves activation of series of molecular events. It is characterized by cell shrinkage, blebbing of plasma membrane, chromatin condensation that consistent with DNA fragmentation and mitochondrial membrane transition [11-15].
In recent studies a great number of phytolectins are reported to have anti- proliferative effect on various human cancer cell lines [16-20]. One of the phytolectin Arisaema intermedium lectin (AIL) has been identified and characterized in our laboratory [21-22]. Therefore the present study was designed to evaluate anti-proliferative effect of Arisaema intermedium lectin (AIL) on HCT-15, a human colon cancer cell line. These studies were perused further to study the me-chanism of action of this lectin by employing various parameters.
MATERIALS AND METHODS
Plant material- The tubers of Arisaema intermedium were collected from Shimla in the month of August, 2013, Department of Molecular Biology and Biochemistry, Guru Nanak Dev University, India.
Isolation of lectin- The tubers were washed and dried. After drying, the tubers were weighed and cut into small pieces. These are mixed with 0.01 M PBS, pH 7.2 in 1:5 ratio (w/v) and homogenized in whirring blender. After blending the slurry in PBS (Phosphate buffer saline) was kept overnight at 4°C. The slurry thus prepared was filtered with surgical gauge. The filtrate was then centrifuged at 12,000 rpm in refrigerated centrifuge (CPR24, REMI) for 20 minutes and the supernatant (crude extract) was collected. It was stored at -20°C till further use.
Hemagglutination assay- 2% erythrocyte suspension of rabbit was used for the determination of lectin activity by hemaglutination assay [23]. The assay was performed in 96 well microtitre plate (Tarsons). To 30 µl of lectin sample, an equal volume of 2% rabbit erythrocyte suspension was added. The plate was incubated for 30 minutes at 37°C and then kept at 4°C overnight. Positive lectin activity was determined by mesh formation, while button formation indicates absence of lectin activity. The titre of crude extract was determined by serial two fold dilution of the sample in 0.01 M PBS, pH 7.2.
Purification and determination of protein concentration- The lectin was purified using asialofetuin linked amino-activated silica beads column according to the already explained protocol [24]. 12 ml of Crude extract containing 16.4 mg of protein was loaded on the col-umn and was re-circulated twice to ensure complete adsorption of lectin molecules. The column was washed with 0.01 M PBS, pH 7.2 for the removal of any unbound molecules. The bound lectin was eluted with 0.1 M Glycine-HCl, pH 2.5 and the fractions were immediately neutralized with 2 M Tris-HCl, pH 8.8. The column was equilibrated with 0.01 M PBS, pH 7.2. The purity of lectin preparation was determined by SDS-PAGE. The SDS-PAGE of the heat denatured lectin sample was performed according to method of Lamelli [25] using 11% (w/v) separating and 5% stacking gel. Electrophoresis was carried out at 25°C at a constant voltage of 90 V in Tris glycine buffer (electrode buffer) pH 8.3, when the tracking dye was in stacking gel and switched to 100 V when it entered the resolving gel. When the tracking dye reached approximately 1.5 cm above the bottom of the gel, the electrophoresis was switched off and the gel was stained with 0.25% Coomassive Blue for 2 h and subsequently destained by gentle agitation on a shaker until the background of bands became clear. Further protein concentration was determined by me-thod [26].
Cell line and Cell culture- Colon cancer cell line HCT-15 (Passage No. 16) was procured from National Centre for Cell Sciences (NCCS) Pune and cells were cultured in RPMI-1640 medium containing 10% FBS. The cell cultures were maintained in CO2 incubator with 5% CO2 and 70-80% humidity.
Cytotoxicity assay- The cytotoxicity of AIL to colon cancer cell line HCT-15 (Colorectal adenocarcinoma) was evaluated with the help of MTT assay to produce formazon crystals [27]. For this, 100 µl of cell suspension at concentration of 8.0 × 103 was added to each well and plates were placed overnight in CO2 incubator at above mentioned condition. The lectin was serially diluted in RPMI-1640 supplemented with 10% fetal bovine serum (FBS), to obtain the desired concentration at a final volume of 100 µl. A negative control was set with cells cultured with RPMI-1640 medium alone. After 42 h treatment with AIL, the medium was removed and 100 µl of MTT solution in RPMI-1640 (0.5 mg/ml) was added. Plates were again incubated in the CO2 incubator (Heraeus, USA) for 2-3 h. The ELISA plate was centrifuged (Centrifuge5804R, Eppendorf) at 2,000 rpm for 2 minutes. The medium was discarded and 100 µl of DMSO was added to each well. The intensity of colour so produced was measured at 540 nm on ELISA reader (Multiscan Ex Labsystem).
Percentage proliferation was calculated by the formula:
DNA fragmentation and Nucleic acid content (NAC) analysis- In DNA fragmentation, apoptosis was assessed by electrophoresis of extracted genomic DNA from treated HCT-15 cell lines and in second assay the number of cells in both the control and treated cell samples were estimated on the basis of their nucleic acid content. Cells were reseeded at concentration of 2.0 × 105 cells/ml in 6-well plates and exposed to different concentration of AIL (100 µg/ml, 50 µg/ml, 25 µg/ml) in for 24 and 48 h. Then, cells were harvested for DNA isolation. Cell suspensions of 2 ml were centrifuged (Centrifuge 5804R, Eppendorf) for 10 minutes at 13,000 rpm. The supernatant was discarded and white pellet was dissolved in 300 µl of WBC’s lysis buffer by vortexing it briefly. After that 25 µl of 10% SDS was added in the tubes. The centrifuged tubes were then incubated for 30 minutes at 56°C and then allowed to cool at room temperature. After cooling, 150 µl of Ammonium acetate was added to the tubes and vortexing was done. Then, the tubes were centrifuged at 13,000 rpm for 10 minutes. Supernatants were collected into fresh tubes and 95% ethanol was added and then spooling was done to precipitate the DNA and centrifuged at 13,000 rpm for 10 minutes. Supernatant was discarded and the pellet was washed with 70% ethanol twice. The pellet was dried at 60°C on the dry bath for 5 minutes. The dried pellet was resupended in 50 µl of TE buffer and incubated at 65°C for 10 minutes to dissolve DNA. The gradation in nucleic acid content was assessed by taking absorbance at 260 nm in treated cells compared to controls on spectrophotometer (Bio Spectrophotometer, Eppendorf) and by electrophoresis using 1.5% agarose gel at 50 V for 2 h. The gel was photographed using alpha imager mini.
Trypan blue exclusion assay - Effect of AIL on viability of HCT-15 cell line was determined with the help of trypan blue exclusion assay. For this, the cells were plated in the 96-well plate at the concentration of 50,000 cells/ml. At 70% confluency cells were treated with various concentra-tion of AIL (100 µg/ml, 50 µg/ml, 25 µg/ml) for 24 h and non treated cells were used as control. The AIL diluted RPMI-1640 was then discarded and 40 µl of non- trypsinized Hank’s was added for two minutes. The concentration of trypsin diluted with the addition of 100 µl of RPMI-1640 so that enzymatic activity can stop. Then the cells were agitated properly and transferred into fresh 15 ml vial centrifugation was done at 1,500 rpm for 5 minutes. After centrifugation, the supernatant was discarded and cells were resuspended in 50 µl of RPMI-1640. Cell suspension of about 10 µl was diluted with 10 µl of RPMI-1640. This diluted cell suspension then mixed with trypan blue at (0.4%) in ratio 1:1. This mixture was kept on neubaur’s hemocytometerand covered with coverslip and observed under microscope at 40X.
The numbers of viable cells per ml of volume were calculated by cell viability formula:
Evaluation of Cell morphology- Effect of AIL on morphology of HCT-15 cell line was observed with the help of phase contrast microscope (Magnus, Olympus). The cells were treated with various concentrations of AIL in time and dose dependent manner. The cells were plated in the 6- well plate at concentration of 1.0x105 cells/ml and non treated cells were used as control. After achieving the 70% confluency, cells were treated with AIL at different concentration i.e. 100 µg/ml, 50 µg/ml, 25 µg/ml for 24 and 48 h. RPMI-1640 added in the cells after rinsing in Non-trypsinized Hank’s solution and photographed using digital camera attached to the mi-croscope.
RESULTS AND DISCUSSION- Apoptosis is main mechanism of cell death caused by lectins as reported in literature [28,14-15]. An important marker of cell death is the disruption of mitochondrial membrane accessed by MTT assay. The present study reports the anti-proliferative potential of AIL on HCT-15 induced by apoptosis. As shown in table 1, the MTT based assay demonstrated that with increasing concentration of AIL, i.e. from 12.5 µg/ml to 100 µg/ml, the percentage of growth inhibition also increased. At the highest dose tested i.e. 100 µg/ml the percentage growth inhibition was 53% after 42 h of AIL exposure. Thus, the detailed analysis of the results clearly indicated that AIL caused significant growth inhibition of HCT-15 cells in dose dependent manner as shown in Fig. 1. Cytotoxicity is potent to induce includes disruption of cytoplasmic and mitochondrial membrane and permeabilize them [29]. These findings are in consonance with the previous reports which showed that lectins inhibit growth of cancer cells in culture conditions as well as in animal models [30-34].
The anti-proliferative effect of AIL further assessed by decrease in nucleic assay content of treated HCT-15 cells in dose dependent and time dependent manner. The decrease of DNA content in gradation showing that as the dose concentration increases there is reduction in DNA content.
Table 1. Cytotoxic effect of A. intermedium lectin on HCT-15 cell line
| Conc. of AIL (µg/ml) | 0 (Control) | 12.5 | 25 | 50 | 100 |
|---|---|---|---|---|---|
| Percentage proliferation ±SD | 100 | 87.9±1.1 | 80.91±0.6 | 92.33±0.3 | 47.23±0.5 |
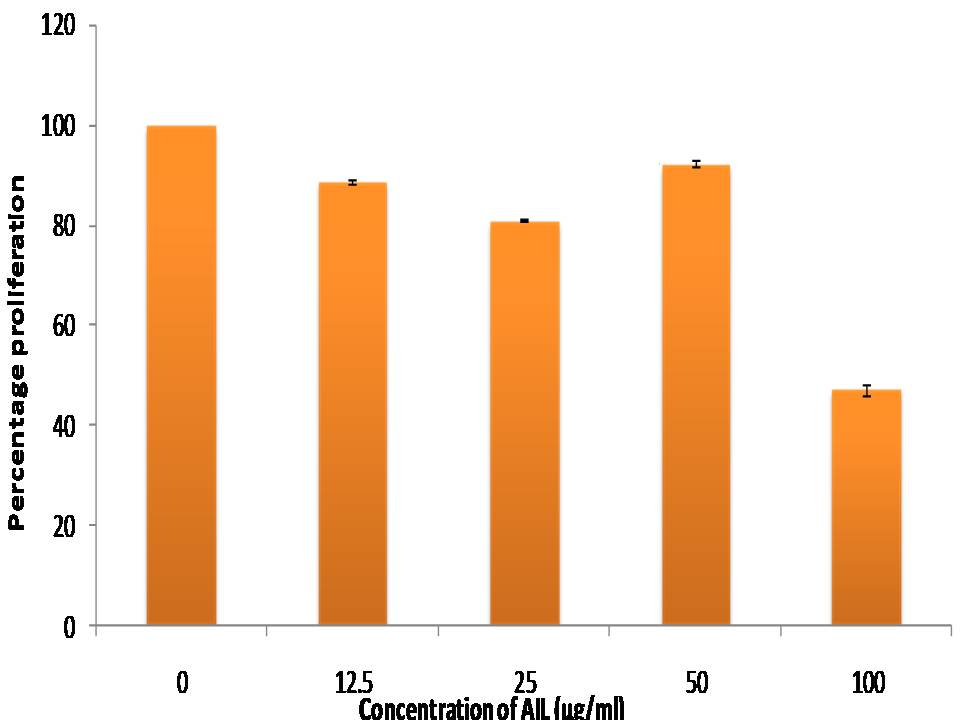
Fig 1. Cytotoxic effect of A. intermedium lectin on HCT-15 cell line: in vitro anti-proliferative potential of AIL towards HCT-15 cell line was evaluated by MTT assay. Bars represent the mean ±SD of percentage proliferation
Table 2. Nucleic acid content measured at different concentrations of AIL for 48 h
| Concentration of AIL (µg/ml) | A260 Mean ± SD | Concentration (ng/µl) Mean ±SD |
|---|---|---|
| 0 (Control) | 0.319± 0.11 (100%) | 2007.75 ±711.98 (100%) |
| 25 | 0.165± 0.053 (51.72%) | 1165.82±185.68 (58.02%) |
| 50 | 0.103± 0.007 (32.28%) | 1038.37±73.75 (51.17%) |
| 100 | 0.079 ± 0.006 (24.76%) | 703.06±77.187 (35.01%) |
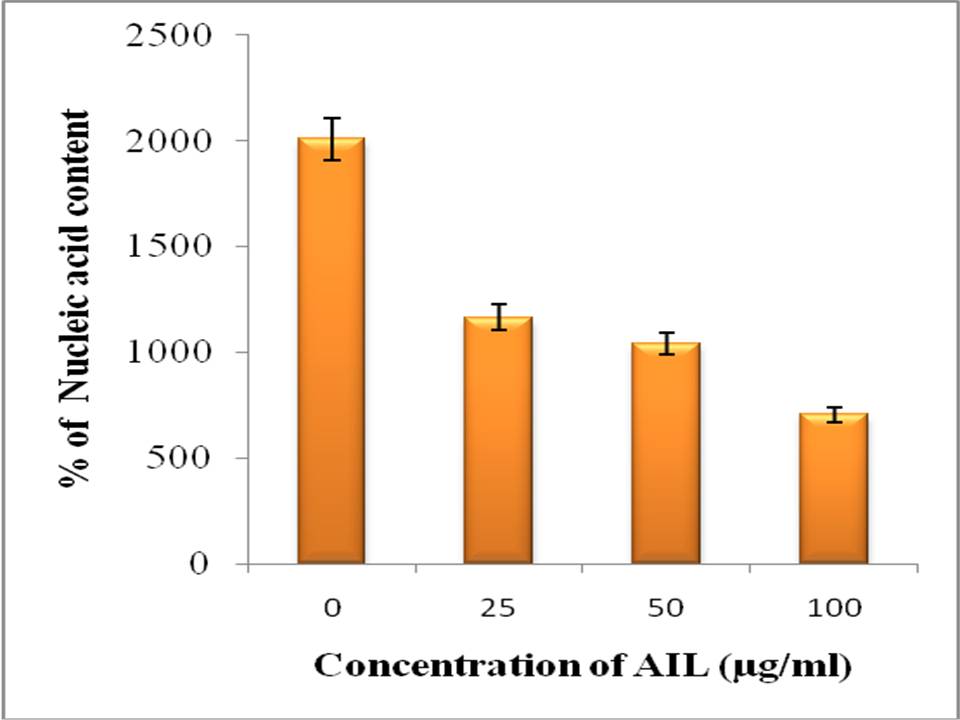
Fig 2: Nucleic acid content analysis: in vitro anti-proliferation was assessed on the basis of nucleic acid content of the treated cells with AIL for 48 h
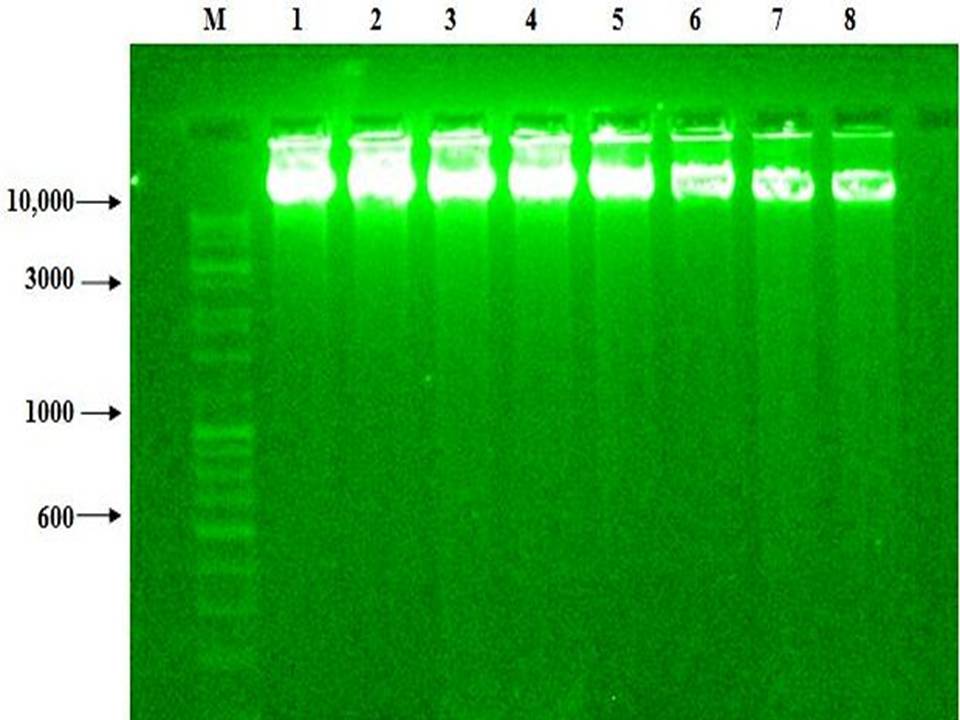
Fig 3: AIL induced DNA fragmentation of genomic DNA: DNA fragmentation was studied in HCT-15 cell line after treatment with different concentration 25, 50 and 100 (µg/ml) of AIL in duplicates for 48 h.
A prominent fragmentation was observed at all doses after 4
| Lane | M | 1, 2 | 3, 4 | 5, 6 | 7, 8 |
|---|---|---|---|---|---|
| Legend (µg/ml) | Marker | 0 (Control) | 25 | 50 | 100 |
Table 4: Cell viability (%) at different concentrations of A. intermedium lectin
| S. No. | Dose conc. (µg/ml) | Viability % |
|---|---|---|
| 1 | 0(control) | 87.50 (100)* |
| 2 | 1.56 | 87.50 (100)* |
| 3 | 3.12 | 80.00 (91.36)* |
| 4 | 6.25 | 78.57 (89.73)* |
| 5 | 12.50 | 75.00 (85.65)* |
| 6 | 25 | 69.23 (79.06)* |
| 7 | 50 | 40.00 (45.68)* |
| 8 | 100 | 28.57 (32.65)* |
The cancerous cells were further studied for the morphological changes induced by AIL. As evident from Fig. 4 (A-G), the AIL treatment produced significant morphological changes in HCT-15 cells in culture. Under normal conditions (control), these cells are adherent and show epithelial like morphology. These cells tend to cluster together and have characteristic pavement like appearance (Fig.4 A). After treatment with 25 µg/ml of AIL for 24 h, although there was no visible change in morphology, the cells started becoming non-adherent. Exposure of cells to 100 µg/ml concentration of AIL for 24 h resulted in complete change in morphology and cells became nearly non-adherent. Furthermore, the extracellular matrix was also found to disappear (Fig. 4 B-D). With increase in exposure time of AIL i.e. from 24 h to 48 h, cells started to loss adherence to cells substratum at 25 µg/ml dose of AIL as observed after 24 h treatment at same dose. Along with this some cell membrane damage was also seen, which was shown to increased with increase in concentration of AIL. At the highest concentration tested i.e. 100 µg/ml. There was appreciable damage of cellular membrane as well as loss of extracellular matrix (Fig.4 E-G). In nut shell, the lectin treatment resulted in lose of integrity, adherence and extracellular matrix along with cell membrane disruption. These events may disrupt the cellular physiology thus leading to cell death. This type of cell morphology disruption of HCT-15 cells has earlier been observed with treatment of various other compounds [38-40].
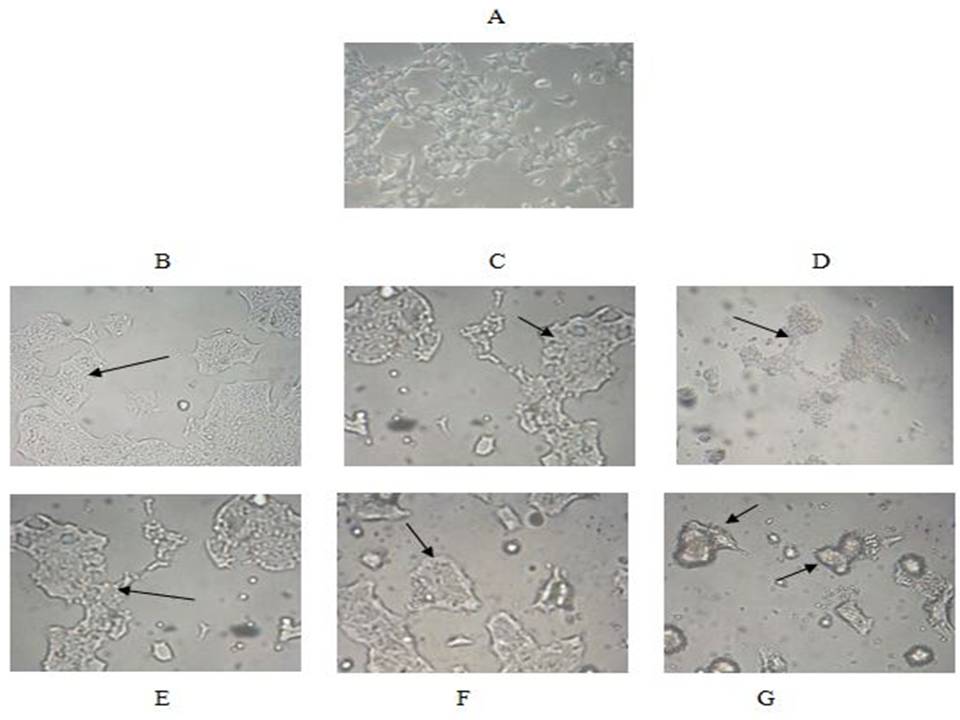
Fig 4. Morphological analysis:
Fig A. Represents normal HCT-15 cell line (Control)
The cells were treated with AIL at 25 µg/ml, 50 µg/ml and 100 µg/ml concentrations for 24 (B-D) and 48 hour (E-G)
REFERENCES
- Segal, Neil H, Saltz, Leonard B. Evolving treatment of advanced colon cancer. MedRev, 2009; 60: 207-219.
- WHO, (Global cancer report) 2011.
- Boyle P, Leon ME. Recent developments in the epidemiology of colorectal cancer. In: Bleiberg, H., Kemeny, N., Rougier, P., Wilke, H. editors. Colorectal cancer: A clinical guide to therapy, chapter 2, UK. Taylor and Francis, 2002; 11–29.
- Lieberman D. Colorectal cancer screening and surveillance. In: Ginsberg GG, Kochman ML, Norton I, Gostout CJ, editors. Clinical gastrointestinal endoscopy, Chapter 37, UK. 2005; Elservier, 537–47.
- Midgley RS, Merrie A, Kerr DJ, Mortensen N. Colorectal cancer: a multidisciplinary approach. In: Weinstein, WM, Hawkey CJ, Bosch J, editors. Clinical gastroenterology and hepatology, Chapter 60D. Spain. Elsevier, 2005; 421–30.
- Xiago-Peng H, Ya-Li Z, Zheng Y, Jia L, Robert A, Guo-Rong C. Carbohydrate CuAAC click chemistry for therapy and diagnosis. Carbohydrate Res, 2016; 429: 1-22.
- Shayli V. Waleed M, Pegah V, Pavla S, Istvan T. Glycosylation, an effective synthetic strategy to improve the bioavailability of therapeutic peptides. Chem Sc, 2016; 7: 2492-2500.
- Greenwald P, Clifford CK, Milner JA. Diet and cancer prevention. Eur J Cancer. 37: 948-65.
- Taylor AD, Hancock WS, Hincapie M, Taniguchi N, Hanash SM. Towards an integrated proteomic and glycomic approach to finding cancer biomarkers. Genome Med, 2009; 1-57.
- Goldstein R, Irwin J, Colin H, Michel M, Toshiaki O, Nathan S. What should be called a lectin. Nature, 1980; 285: 66.
- Robertson JD, Orrenius S. Molecular mechanism of apoptosis induced by cytotoxic chemicals. Crit REV Toxicol, 2000; 30: 609-627.
- Gupta S. Molecular steps of death receptor and mitochondrial pathways of apoptosis. Life sci, 2001; 69: 2957-2964.
- Chen M, Wang J. Initiator caspase in apoptosis signaling pathways, Apoptosis. 2002; 7: 313-319.
- Jiang L, Zhang S, Tian M, Zhang S, Xie T, Liu L, Quyang L. Plant lectins from ancient sugar- binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Proliferation, 2015; 48: 17-28.
- Zheng S, Rong S, Tian Y, Rong L, Jin-Ku B, Lian Z, Yong T. Identification Of novel pathways in plant lectin-induced cancer cell apoptosis. Molecular Sciences, 2016; 17(2): 1-15.
- Anagh A, Sahasrbudhe N, Ahmed MV, Krishnasaatry, Stress-induced phosphorylation of caveolin-1 and p38, and down-regulation of EGFr and ERK by the dietary lectin jaclin in two human carcinoma cell lines. CSSI, 2006; 11 (2): 135-147.
- Oliveira C, Nicolau A, Jose A, Teixeira, Lucilia D, Cytotoxic effects of native and recombinant Frutalin, a plant Galactose-Binding lectin, on HeLa Cervical Cancer Cells. J Biomed Biotech, 2011; 568932: 1-9.
- Sang Y, Tzi N. A lectin with highly potent inhibitory activity toward breast cancer cells from edible tubers of Dioscorea opposite Nagaimo). Biomeducal Sciences, 2013; 8: 1-11.
- Monira P, Koyama Y. Mamoru I, Yoriyuki N. Plant lectin in therapeutic and diagnostic cancer research. Int J Biol Res, 2015; 3(2): 1030.
- Tammy Y, Xiuli D, Charlene N, Tzi N. Lectins with potential for anti- cancer therapy. Molecules, 2015; 20: 3791-3810.
- Jiahn L, Chin-Ta C, Han-Yung W, Kai-Fa H, Iren W, Meng H. A multivalent marine lectinfrom Crenemytilus grayanus Possesses Anti-cancer activity through recognizing globotriose Gb3. J. Am. Chen Soc, 2016; 138: 4787-4795.
- Lavanya V. Mohmmad Adil A, Ahmed N, Jamal S. Lectin the promising cancer therapeutics. Oncobiology, 2016; 1: 242-259.
- Kaur M, Singh J, Kamboj SS, Singh J, Kaur A, Sood SK, Saxena AK. Isolation and characterization of the N-acetyl- D-lactosamine specific lectins from tubers of Arisaema intermedium Blume and A. wallichianum Hook. Indian J. Biochemestry and Biophysics, 2005; 42: 34-40.
- Kaur M, Singh K, Rup PJ, Kamboj SS, Singh J. Anti-insect potential of lectin from Ariseama intermedium species towards Bactrocera cucurbitae. J Environ Biol, 2009; 30 (6): 1019-1023.
- Lamelli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 1970; 277: 680-685.
- Lowry OH, Rosebrough NJ, Farr AL, Ranald RJ. Protein measurement with the folin Phenol reagent. J Biol Chem, 1951; 193: 265-275.
- Mosmann T. Rapid calorimetric assay for cellular growth and survival application to proliferation and Cytotoxic assays. J. Immuno. 1983 65: 55-63.
- Faheina-Martins GV, Silveira AL, Marques LF. Influence of fetal bovine serum on Cytotoxic and gonotoxic effects of lectins in MCF-7 cells. J Biochem. Mol Toxic, 2012; 25: 290-296.
- Bakkali F, Averbeck SD, Idaomar M. Biological effects of essential oils. Food Chem Toxicol Rev, 2008; 46: 446-457.
- Kiss R, Canby I, Duckworth De. R, Decker, Salmon I, Pasteels JL, Danguy A, Yeaton P. in vitro influence of Phaseolus vulgaris, Griffonia simplicifolia, Concanavalin A, Wheagerm, and peanut agglutinin on HCT-15, LoVo, and SW837 human colorectal cancer cell growth. Gut, 1997; 40: 253-261.
- Xiu YJ, Tzi B, Antitumor and HIV-1 reverse transcriptase inhibitory activities of a hemagglutination and a protease inhibitor from Mini-Black soyabean. J Biomed , 2011; 851396: 1-12
- Lu Q, Li N, Luo J, Yu M, Huang Y, Wu X, Wu H, Liu XY, Li G. Pinellia pediatisecta agglutinin interacts with the methylosome and induces cancer cell death. Oncogenesis, 2012; 12: 2157-9024.
- Chenjiing S, Quishi C, Anne D, Stuart H, Winnock H, Els D. The cytotoxicity of Elderberry ribosome- inactivating proteins is not solely determined by their protein translation inhibition activity. Primljeno, 2015; 51: 211-229.
- Ruby S, Laxman N, Dhiman S, Suresh CG. Two chitotriose lectins show anti-angiogenesis, induces caspase 9 Mediated apoptosis and early arrest of pancreatic tumor cell cycle. PLOS, 2016; 11: 1-18.
- Wylie AH. Glucocorticoi-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature, 1980; 284: 555-556.
- Liu B, Li CY, Bian HJ. Antiproliferative activity and apoptosis inducing mechanism of Concanavalin A on human melanoma A375 cells. Arch Biochem. Biophys, 2009; 482: 1-6.
- Ingale AG, Hivrale AU. Plant as a plenteous reserve of lectin. Lands Biosciences, 2013; 8: 1-13.
- Devi SP, Kumar SM, Das SM. Evaluation of Anti-proliferative activity of red sorghum bran anthocyanin on a human breast cancer cell line MCF-7. J Breast, 2011; 23: 123-127.
- Tavakolinia F, Taybeh B, Hossaini Z, Daryoush Z, Mohammad AK, Mehdi R. Antiproliferative activity of noval thiopyran analogs on MCF-7 breast and HCT-15 colon cancer cells: synthesis, cytotoxicity, cell cycle analysis and DNA-binding. Cancer, 2012; 10: 1-8.
- Jasminka G. Plant lectins in cancer prevention and treatment. Medicina fluminensis, 2015; 51: 211-229.
| Source of Financial Support: Nil Conflict of interest: Nil |
|---|
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/ |
|---|