Materials and Methods: During the study period, ethological responses in the exposed fish (240 in number irrespective of sex) were observed. Changes in some haematological parameters like haemoglobin content (Hb), haematocrit (Ht), total erythrocyte count (TEC), mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH), mean corpuscular haemoglobin concentration (MCHC) and blood glucose (Glu) were also measured in the exposed fish (60 in number irrespective of sex) during the acute toxicity test at different time interval.
Results: The 24, 48, 72 and 96 h LC50 values for C. gachua were 21.80, 19.59, 13.95 and 11.18 g/l respectively. Toxicity factor values of this phytopiscicide to the fish were increased with the progress of exposure time. They showed an alteration in their responses with the increasing concentration of neem leaf extracts and time of exposure. A significant elevation in the level of blood glucose in the exposed fish throughout the exposure period was recorded while Hb showed significant change after 24 h and that of MCHC only at 96 h.
Conclusions: The study may help to determine the toxic level of the aqueous extracts of neem leaves to the unwanted fish in aquaculture farm and to understand the mode of its action to fish behaviour and haematology.
Key-words- Acute toxicity, Azadirachta indica, Snake Headed Fish, Haematological Parameters, Ethological Responses
INTRODUCTION- Neem (Azadirachta indica A. Juss; Family: Meliaceae), a widely distributed tree in India, has been used medicinally since long back and presently emphasis has been given on its biopesticidal properties1-2. It has also been suggested as an alternative phyto-piscicide by some earlier workers3-7 besides its extensive use in fish farms to control fish parasites and fish fry predator insects8-9.
Its piscicidal potential is embedded in the alkaloids that may cause functional changes in biochemical activities and organ morphology of the fish species3, 5-6, 10-11.
Therefore, the objective of the present study was to determine the acute toxicity of neem leaf extract to the snake headed fish, Channa gachua (Ham.) as they are commonly eradicated from the fish pond as unwanted ones prior to stocking during scientific aquaculture practice. Their ethological responses and changes in some haematological parameters were also evaluated in the present study during bioassay to find the mechanism of toxic action.
MATERIALS AND METHODS- Healthy and disease free fish, Channa gachua (mean length 15.86 ± 1.26 cm, mean weight 19.0 ± 1.21 g) procured from local market was used in the bioassay in the laboratory, Department of Zoology, Jhargram Raj College, Paschim Medinipur, West Bengal during July-August, 2014. The fish were treated with 0.1% KMnO4 solution to avoid any pathogenic infection and acclimatized to the test condition for a week before their use.
Fresh leaves from neem plant (Azadirachta indica) were collected locally and cleansed with de-chlorinated tap water to remove dust. Washed leaves were air dried at room temperature, chopped and finely grounded in blender. To prepare an aqueous extract the dried and grounded leaves were soaked in distilled wa-ter for 24h at room temperature at a concentration of 25g of dried leaves per litre of water12. The mixture was filtered and the extract (25g/l) was used immediately for the experiment at different concentrations.
Static replacement bioassays were used for both acute toxicity tests for determination of 24, 48, 72 and 96h LC50 and ethological study. The underground water (temperature 27 ± 0.45 °C, pH 7.4 ± 0.21, free CO2 8.0 ± 0.21 mg/l, DO 5.54 ± 0.42 mg/l, alkalinity 176 ± 7.01 mg/l as CaCO3, hardness 120 ± 7.0 mg/l as CaCO3) was used as a test medium. The test medium was replaced every 24h by freshly prepared test solution to avoid the interference of different abiotic factors with the animals’ performance. Water chemical analysis and the bioassays were done following the methods outlined by American Public Health Association13.
Acute toxicity tests for fish irrespective of sex were conducted in 45l glass aquaria holding 30l of water in the laboratory. The selected test concentrations of neem leaf extract used for the determination of acute toxicity to Channa gachua were 0, 6, 8, 10, 12, 14, 16, 18 and 20 g/l based on rough range finding tests. Each concentration was accompanied by three replicates. Ten organisms were used in each replicate. The fishes were not fed 24h before and during the bioassays. The number of dead fishes was counted every 24h and removed immediately from the test medium to avoid any organic decomposition and oxygen depletion. Mortality rate of C. gachua at different concentrations at every 24h of exposure was analyzed to estimate 24, 48, 72 and 96h lethal concentrations (LC1, 10, 50, 90, 99 values) with 95% confidence limits using the computer software R version 2.14.014 and probit analysis15. On the basis of acute toxicity values, toxicity factors at different exposure period were assessed16.
The ethological changes in activity, body balance, rate of swimming, fin and opercular movement, and body movement pattern followed by death of the fish exposed to different lethal concentrations of the neem leaf extracts were recorded at 24, 48, 72 and 96 h17.
A group of healthy, active and properly acclimatized 15 fishes was exposed to 96h LC50 concentration of neem leaf extract for acute toxicity tests to determine the ef-fects on fish blood profiles. Control fishes were main-tained under identical conditions without toxicant in the medium. Three replicates for each of the treatment and control were arranged. Three fishes were from each aquarium were sacrificed at 24, 48, 72 and 96h. Fish were randomly collected from both control and treatment, anaesthetized with 1:4000 MS222 (tricane methane sulphonate; Sandoz). Blood was drawn by severance of caudal peduncle and was collected in non-heparinized Eppendorf tubes using 0.02 ml of 10% EDTA (dipotassium salt of ethylene diamine tetra acetic acid; Sigma) as anticoagulant. Haemoglobin content (Hb), haematocrite (Ht), total erythrocyte count (TEC) and other erythrocyte indices like mean corpuscular volume (MCV), mean corpuscular haemoglobin (MCH) and mean corpuscular haemoglobin concentration (MCHC) were estimated following the standard methods18-19. Blood glucose content in control and treated fish blood without EDTA was estimated by the assay kit following Enzymatic-Colorimetric method20.
The mean values of haematological parameters for the control and treated fish were subjected to student’s‘t’ test to determine significant differences among the means.
RESULTS- The lethal concentrations (LC1,10,50,90,99) of neem leaf extracts at different time of exposure (24, 48, 72 and 96h) with 95% confidence limits and toxicity factors to Channa gachua are given in Table 1and 2. No mortality was observed in the control group during the experiment.
The ethological changes observed in fishes exposed to neem leaf extracts were recorded in Table 3. The res-ponses to the toxicant in the form of alteration in beha-viour increased with the dose and duration. In the present study the exposed fish showed erratic swimming, loss of equilibrium and hyperactivity with the advancement of time of exposure and concentration. Initially rapid fin movement and swimming rate of the treated fish was gradually decreased and ultimately stopped at 96h of exposure at higher concentrations of neem leaf extracts. Their normal jumping behaviour and somersaulting activities ceased with the progress of time. Treated fish also exhibited various distressed signs like reduced movement, bottom settling in a motionless condition and threadlike mucous secretion from vent at 96h of exposure at higher concentration of neem extracts. But opercular movement of the exposed fish did not affect severely (Table 3).
Table 1. Acute lethal concentrations (LC) with 95% confidence limits of neem leaf extracts to Channa gachua (Control group theoretical spontaneous response rate= 0.0000)
| Lethal concentrations (g/l) | 24h | 48h | 72h | 96h |
|---|---|---|---|---|
| LC1 | 9.23 (2.44-11.93) | 6.43 (1.94-8.96) | 4.50 (1.79-6.44) | 3.78 (1.61-5.44) |
| LC10 | 13.58 (8.43-15.74) | 10.60 (6.28-12.73) | 7.48 (4.47-9.30) | 6.15 (3.66-7.78) |
| LC50 | 21.80 (18.34-36.57) | 19.59 (16.44-31.56) | 13.95 (11.94-16.79) | 11.18 (9.33-12.96) |
| LC90 | 36.21 (25.31-63.12) | 34.99 (24.92-52.74) | 26.01 (20.32-47.56) | 20.32 (16.70-30.71) |
| LC99 | 51.46 (31.46-79.93) | 49.73 (35.01-72.40) | 43.24 (29.21-59.22) | 33.07 (24.03-68.29) |
Table 2. Toxicity factor of neem leaf extracts to Channa gachua at LC50 value under different exposure periods
| Exposure time (h) | Toxicity factor value |
|---|---|
| 24 | 1.00 |
| 48 | 1.11 |
| 72 | 1.56 |
| 96 | 1.95 |
Table 3. Impact of neem leaf extracts on ethological responses of Channa gachua at different time of exposure and concentrations (none: -; mild: +; moderate: ++; strong: +++)
| Ethological parameters | Exposure time (hr) | Concentrations of neem leaf extracts (g/l) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0(control) | 6 | 8 | 10 | 12 | 14 | 16 | 18 | 20 | ||
| Fin movement | 24 | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | + |
| 48 | +++ | +++ | ++ | ++ | ++ | ++ | + | + | + | |
| 72 | +++ | ++ | ++ | ++ | + | + | + | + | + | |
| 96 | +++ | ++ | + | + | + | + | + | + | - | |
| Opercula movement | 24 | +++ | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ |
| 48 | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | |
| 72 | +++ | +++ | +++ | ++ | ++ | ++ | ++ | + | + | |
| 96 | +++ | +++ | +++ | ++ | ++ | ++ | + | + | + | |
| Jumping movement | 24 | ++ | ++ | ++ | ++ | ++ | +++ | +++ | +++ | +++ |
| 48 | ++ | ++ | ++ | +++ | +++ | +++ | +++ | ++ | ++ | |
| 72 | ++ | ++ | +++ | +++ | +++ | ++ | ++ | + | - | |
| 96 | ++ | +++ | ++ | ++ | + | - | - | - | - | |
| Swimming rate | 24 | +++ | +++ | +++ | +++ | ++ | ++ | ++ | + | + |
| 48 | +++ | +++ | +++ | ++ | ++ | ++ | + | + | + | |
| 72 | +++ | +++ | ++ | ++ | ++ | ++ | + | - | - | |
| 96 | +++ | +++ | ++ | ++ | ++ | ++ | + | - | - | |
| Somersaulting activity | 24 | +++ | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ |
| 48 | +++ | +++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | |
| 72 | +++ | ++ | ++ | ++ | + | + | + | + | + | |
| 96 | +++ | +++ | + | + | + | - | - | - | - | |
| Equilibrium status | 24 | +++ | +++ | +++ | +++ | +++ | +++ | ++ | ++ | + |
| 48 | +++ | +++ | ++ | ++ | ++ | ++ | ++ | ++ | + | |
| 72 | +++ | +++ | ++ | ++ | ++ | ++ | + | + | + | |
| 96 | +++ | ++ | + | + | + | + | + | - | - | |
| Staying period | 24 | - | - | - | - | + | + | + | ++ | ++ |
| 48 | - | - | - | + | + | + | + | ++ | ++ | |
| 72 | - | - | + | + | + | + | ++ | +++ | +++ | |
| 96 | - | - | + | + | ++ | ++ | +++ | +++ | +++ | |
| Mucous secretion | 24 | - | - | - | - | - | - | - | - | - |
| 48 | - | - | - | - | - | - | - | - | - | |
| 72 | - | - | - | - | - | - | - | - | - | |
| 96 | - | - | - | - | - | - | - | + | + | |
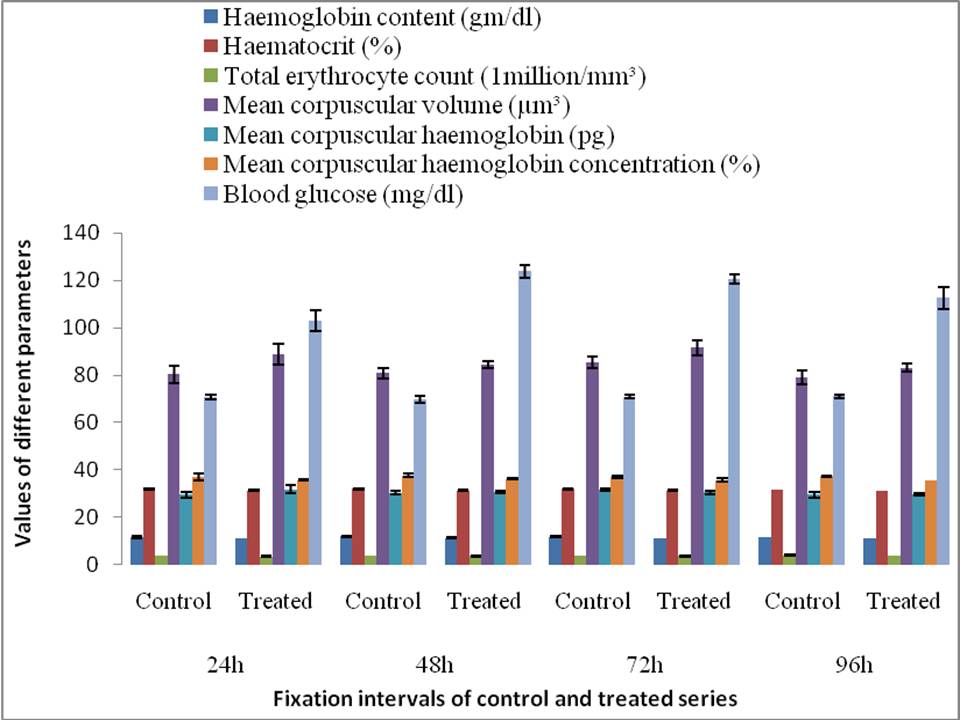
Changes in haematological parameters in exposed fish at different times during acute toxicity study were summa-rized in Fig.1. The present study demonstrated that the fish exposed to neem leaf extracts displayed a significant elevation in the level of blood glucose throughout the exposure period over the control. But haemoglobin content showed significant changes at 48, 72 and 96 h, whereas mean corpuscular haemoglobin concentration showed significant changes only at 96 h.
DISCUSSION- The acute toxicity of neem leaf extracts to fish is very much pervasive5. The toxic effects of neem leaf ex-tracts to fish C. gachua expressed as 24h LC50 values found in the present observation (21.8 g/l) is manifold higher than the earlier findings7,21. Similarly, 96h LC50 value for O. mossambicus (5.83 g/l) and Clarias gariepinus (4.00 g/l) as recorded earlier4,7 were also lower than the present findings (11.18 g/l). Such variation in the lethal toxicity is probably due to variation in fish species used, their age, sex and size, test methods and water quality7, 21-22. Further, higher median lethal concentration (LC50 value) for C. gachua in the present study may be attributed to their hardy nature and activeness to cope up with stress condition for being air-breathing fish23. Differences in the sensitivity of fish species to neem may also be related to the variation in the amount of active compound present in neem depending on its plant parts, its origin or even the individual tree24-25. With the progress of time of exposure toxicity factor for neem leaf extracts as a toxicant increases to C. gachua in the present investigation which is in conformity to the results obtained from O. mossambicus7.
The study revealed that the neem leaf extracts can cause marked ethological changes in fish and thus demon-strated a sensitive indicator of physiological stress in fish26. It is an indicative of internal disturbances of the body functions due to toxicant induced cumulative deleterious effects at various metabolic sites of the fish body or due to disruption of nervous system function26. Impairment in neural transmission, nervous impairment due to blockage of nervous transmission between the nervous system and various effecter sites, induction of oxidative stress, and disturbances in enzyme mediated metabolic pathways may cause the behavioural responses of the fish to piscicidal toxicity17, 26. Time and dose dependent respiratory distress, erratic swimming and nervous manifestations as recorded in the present study were in conformity with the observation of some earlier workers4,7,21 in different freshwater fish exposed to neem leaf extract. Initial in-crease in jumping, somersaulting activity and increased swimming rate in fish were probably early indication of their avoidance reaction from the toxicant which may be related to narcotic effects or to change in sensitivity of chemo receptors27. With the progress of time and increasing concentration the fish exhibited sluggish movement and cessation of swimming indicating the effects of neem on the central nervous system. The active ingredient present in the neem probably interfere with the membrane transport of Na+, K+, Ca2+ or Cl- ions, inhibit selective enzyme activities, and contribute to the release and/ or the persistence of neurotransmitters at synaptic junctions which leads to hyperactivity, swimming in imbalanced manner and lethargy28.
The levels of blood glucose in C. gachua were signifi-cantly increased from the corresponding control values throughout the experimental periods when exposed to neem leaf extracts indicating a typical stress response to the increased rate of glycogenolysis or gluconeogenesis29-30. This result is consistent with the earlier findings3 on freshwater fish, Prochilodus lineatus. Such hyperglycaemic condition in fish under acute toxicity of aqueous extracts of neem leaves may be due to impairment in carbohydrate metabolism31. Probably neem leaf extracts as stressor stimulates the adrenal tissue, resulting in increased level of circulating glucocorticoids and catecholamines on the glucose release from the liver, the main carbohydrate store in fish. Both of these two groups of hormones produce hyperglycaemia29-30, 32-33. Such significant increase of glucose induced hyperglycaemia in fish might have resulted to provide energy for the increased metabolic demands to cope up with stress3, 5. Neem extracts did not interfere with the haematopoietic activities in the exposed fish as there were no significant variations in haemoglobin content, haematocrit, total erythrocyte count and other erythrocyte indices. No marked change in opercular movement during ethological study in exposed fish may also correlate with the fact.
CONCLUSION- The present findings highlight the toxicity of neem leaf extracts to fish indicating its potentiality to eradicate unwanted fish from the fish pond. The lethal toxicity values of the extracts to C. gachua, in the present study, thus serve as baseline information on its toxicity which may be helpful in formulating the dose of neem extracts as organic piscicide in aquaculture management. The ethological responses in fish treated with neem leaf extracts provide new vistas to assess the nature of toxicity as well as physiological state of the fish under exposure. The biochemical pathway of the active principle of neem is poorly understood2. But on the basis of haematological findings in the present investigation, it would be possible to forecast the mechanism of action of neem to fish. The toxicity factor recorded in the present study may be used as tool to establish toxicity scale for neem leaf extracts as well as to establish its environmental safety limit in the fish farm for con-trolled management practice.
ACKNOWLEDGMENT- The authors are grateful to Dr. Amalendu Jana, the then Head, Department of Zoology, Jhargram Raj College, Paschim Medinipur, West Bengal for allowing us to carry out the experiment.
REFERENCES
- Biswas K, Chattopadhyay I, Banerjee, RK and Bandyopadhyay, U. (Biological activities and medicinal properties of neem (Azadirachta indica)). Cur Sci, 2002; 82: 1336-1345.
- Rao NDR and Chary P. (Ancient nimba is the power plant of the 21st century). Science and Culture, 2009; 75(1-2): 8-11.
- Winkaler EU, Santos TR, Machado-Neto JG and Martinez CB. (Acute lethal and sublethal effects of neem leaf extract on the neotropical freshwater fish Prochilodus lineatus). Comp Biochem Physiol C Toxicol Pharmacol, 2007; 145(2): 236-244.
- Mousa MAA, El-Ashram AMM and Hamed M. (Effect of neem leaf extract on freshwater fishes and zooplankton community). Proceedings of the 8th International Symposium on Tilapia in Aquaculture, Central Laboratory for Aquaculture Research, October 12-14, Cairo, Egypt, 2008: 307–318.
- Saravanan M, Ramesh M, Malarvizhi A and Petkam R. (Toxicity of neem leaf extracts (Azadirachta indica A. Juss) on some haematological, ionoregulatory, biochemical and enzymological parameters of Indian Major Carp, Cirrhinus mrigala). Journal of Tropical Forestry and Environment, 2011; 1(1): 14-26
- Fafioye OO. (Acute and sub-acute toxicities of five plant extracts on white tilapia, Oreochromis niloticus (Trewavas)). International Research Journal of Agricultural Science and Soil Science, 2012; 2(13): 525-530.
- Dhara K, Biswas SJ and Roy Karmakar S. (Study on the toxicity of neem (Azadirachta indica A. Juss) leaf extracts as phytopiscicide on three life stages of Mozambique tilapia (Oreochromis mossambicus Peters) with special reference to their ethological responses). Int J Exp Res Rev, 2016; 3: 7-13.
- Gáradi P, Domarco RC and Pinheiro CWL. (Avaliação do uso de inseticida (orgânicos fosforados) no combate as Odonatas e na seleção zooplanctônica em piscicultura de al-evinagem). Estudos de Piscicultura, 1988; Brasília, Brazil.
- Martinez SO. (NIM-Azadirachta indica: natureza, usos múltiplose produção). Instituto Agronômico do Paraná (IAPAR), 2002; Londrina, PR.
- Saravanan M, Vasantha Kumar D, Malarvizhi A and Ramesh M. (Biosafety of Azadirachta indica (A. Juss) leaves extracts on certain biochemical parameters of Labeo rohita). Journal of Biopesticides, 2010; 3(1 Special Issue): 227 – 231.
- Alam A, Tabinda AB, Mahmood-ul-Hassan and Yasar A. (Comparative toxicity of acetamiprid and Azadirachta indica leave extract on biochemical components of blood of Labeo rohita). Pakistan J Zool, 2014; 46 (6): 1515-1520.
- Cruz C, Machado-Neto JG, Menezes ML. (Toxicidade aguda do inseticida Pration metilico e do biopesticida azadiractina de folhas de neem (Azadirachta indica) para alevino e juvenil de pacu (Piaractus mesopotamicus). Pesticidas: R Ecotoxicol e Meio Ambiente, 2004; 14: 92-102.
- APHA. Standard methods for the examination of water and wastewater. Rice EW, Baird RB, Eaton AD and Clescen, LS (Eds.) American Public Health Association, American Water Works Association (AWWA) and Water Environment Federation (WEF), 2012; Washington DC.
- US EPA. Probit program version 1.5. Ecological Monitoring Research Division, Environmental Monitoring Systems Laboratory, US Environmental Protection Agency, 1999; Cincinnati, Ohio 45268. http://www.epa.gov/nerleerd/stat2.htm.
- Finney PJ. Probit analysis, 3rd ed., Cambridge University Press, 1971; pp. 333-337.
- Ayoola SO, Kuton MP, Idowu AA and Adelekun AB. (Acute toxicity of Nile tilapia (Oreochromis niloticus) juveniles exposed to aqueous and ethanolic extracts of Ipomoea aquatica leaf). Nature and Science, 2011; 9(3): 91-99.
- Rand GM. Behavior. In: Fundamentals of Aquatic Toxicology Methods and Applications, Rand GM and Petrocelli SR (Eds), Hemisphere Publishing Corporation, Washington, 1985; pp. 221-262.
- Campbell TW and Murru F. An introduction to fish haematology. Compendium of continuing education in veterinary science, 1990; 12: 525-533
- Dacie SJ and Lewis SM. Practical Haematology. Churchill Livingstone, Edinburgh, UK, 1975.
- Trinder P. (Determination of glucose concentration in the blood). Ann Clin Biochem, 1969; 6: 24.
- Cagauan AG, Galaites MC and Fajardo LJ. Evaluation of botanical piscicides on Nile tilapia Oreochromis niloticus L. and Mosquito fish Gambusia affinis Baird and Garard. Proceedings on ISTA, 12-16 September, Manila, Phillipines, 2004: 179-187.
- Kaviraj A and Das BK. (Bioaccumulation and toxicity of cadmium to aquatic organisms- A review). Growth, Development & Natural Resources Conservation, 1990; 3: 177-186.
- Bhattacharya S. Possibilities and magnitude of heavy metal pollution in aquaculture. In: Nutrient Management in Aquaculture, Chattopadhyay GN (Eds), Institute of Agriculture, Palli Siksha Bhavana, Visva Bharati, Sriniketan, India, 1995: 90-92.
- Isman MB, Koul O, Luczyski A and Kaminski J. (Insecticidal and antifeedant bioactivities of neem oils and their relationship to azadirachtin content). J Agric Food Chem, 1990; 38: 1406–1411.
- Lue X, Ma Y, Wu S and Wu D. (Two novel azadirachtin derivates from Azadirachta indica). Journal of Natural Products, 1999; 62: 1022-1024.
- Åkerblom N. Agricultural pesticide toxicity to aquatic organisms- a literature reviews. Miljöanalaya, Department of Environmental Assessment, Swedish University of Agricultural Sciences, Uppasala, Rapport, 2004:16.
- Suterlin AM. (Pollutants and chemicals of aquatic animals perspective). Chem Senses Flavour, 1974; 1: 167-178.
- Ecobichon DJ. Toxic effects of pesticides. In: Casarett and Doull’s Toxicology- The basic science of poisons, Amdur et. al. (Eds). 4th ed., McGraw-Hill, Inc., New York, 1991; pp. 565-622.
- Al-attar AM. (Biochemical effects of short-term cadmium exposure on the freshwater fish, Oreochromis niloticus). J Biol Sci, 2005; 5(3): 260-265.
- Jha SK, Kumari M and Jha MM. (Biochemical changes of mercury chloride on blood metabolite levels of Labeo rohita (Linn.)). Indian J Environ & Ecoplan, 2010; 17(3): 375-378.
- Wedemeyer GA and McLeay DJ. Methods for determining the tolerance of fishes to environmental stressors. In: Stress and Fish, Pickering AD (Eds). Academic Press, London, 1981; pp. 247-275.
- Pickering AD. Stress and compensation in teleostean fishes. Response to social and physical factors, In: Stress and Fish, Pickering AD (Eds), Academic press., New York/London, 1981; pp. 295-322.
- Nath K and Kumar N. (Hyperglycaemic response of Heteropneustes fossilis exposed to nickel). Acta Hydrochem Hydrobiol, 1988; 16: 333-336.
| Source of Financial Support: Nil Conflict of interest: Nil |
|---|
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/ |
|---|