ABSTRACT-
Proteases are class of proteolytic enzymes that accounts for approximately 45% of the total enzyme sales in various pharmaceuticals industrial ranging from potential agent for curing AIDS, treatments of inflammation and virulent wounds, and remodelling of various proteins. Protease has a great boon in food industry, leather industry, and biomining of metals like silver from ores along with bioremediation activity of waste. Microbes serve as a preferred source for proteases and major the contributor to this is derived from Bacillus strains. Bacillus subtilis belongs to this class is the major producer of proteases enzyme commercially. The major issue being the high cost of substrate that makes the overall production cost highly expensive. An attempt was made to formulate media using varied carbon sources to optimize media to maximize the production of proteases. Lactose was found to be an ideal carbon source among the selected carbon sources that yielded maximum biomass of 160 mg/ml. Sucrose with a yield of 132.5 mg/ml and glycerol 129.3 mg/ml which was comparatively less then lactose while the other carbon sources like maltose, glucose, cellulose, starch were found to be poor in comparison to lactose.
Key-words- Proteases, Bacillus subtilis, optimize media, Lactose
INTRODUCTION-
Bacillus subtilis is a naturally occurring saprophytic bacterium that is ubiquitous and can be recovered from soil, water, air, and decomposing plant material. Under unfavourable conditions, it remains inactive in form of spores. An important biotechnological application of protease is in bioremediation processes [1]. Bacillus subtilis are considered free of endotoxin and have GRAS (generally regarded as safe) status. [2] Different strains of B. subtilis are used as biological control agents like the lipopeptide iturin as an antibiotic. [3] Further Bacilus subtilis is widely used bacteria mass production of chemicals and enzymes for industrial use. Bacilus subtilis is a major source for commercial production of amylase and protease enzymes. [4]
Proteases or proteolytic enzymes catalyze the cleavage of peptide bonds in proteins. The extracellular proteases are of commercial value and find multiple applications in various industrial sectors. [5] Protease are the single class of enzymes which occupy a pivotal position due to their wide applicability in detergent, pharmaceutical, photography, leather, food and agricultural industries. It is also used in meat tenderization, baking, brewing, peptide synthesis, medical diagnosis, cheese making, certain medical treatments of inflammation and virulent wounds and in tanning of sheepskins. [6-7] They conduct highly selective and specific modification of proteins i.e. zymogenic form of enzymes by limited proteolysis, processing and transport of secretory proteins across the membrane, blood clotting and lysis of fibrin clots. They catalyze important proteolytic steps in tumor invasion and also play a crucial role in circumvent the infection cycle of a major pathogenic microorganisms. They role in the life cycle of disease causing organisms is also being studied as a therapeutic agents against fatal disease such as cancer and AIDS. [8] At present, the overall cost of enzyme production is very high (due to high cost of substrates and mediums used for mass production) and therefore, development of novel processes to increase the yield of proteases with
respect to their industrial requirements coupled with lowering down the production cost is highly appreciable from the commercial point of view.
This paper aims to formulate media for overall improvement in cost of production of proteases from Bacillus subtilis by standardizing the best carbon sources for maximum yield of proteases in media.
MATERIALS AND METHODS:
Screening and Isolation of proteases producing Bacillus subtilis-
A) Collection of soil sample-
Soil samples were collected on December, 2015 from agricultural fields of Lalitpur district of Uttar Pradesh, India. Serial dilution of 10-5 and 10-8 were spread onto NAM (Nutrient agar media) and incubated at 35°C for 48 hours followed by pure culture technique.
B) Identification of bacteria-
Six strains of Bacillus subtilis namely BS-1, BS-2, BS-3, BS-4, BS-5 and BS-6 were isolated and identified based on cellular morphology, growth condition, gram staining, endospore staining, capsule staining and biochemical tests namely antibiotic sensitivity (penicillin), citrate hydrolysis, motility, Methyl Red- Voges-Proskauer MRVP, Indole production, catalase test, nitrate reduction, gelatine hydrolysis test and production of H2S were performed and Gram-positive, rod-shaped, spore forming bacilli were selected. [9]
C) Identification of bacterial strain for protease production:
For protease screening, casein agar medium (g/l) (peptic digest of animal tissue, 5.0; beef extract, 1.5; yeast extract, 1.5; sodium chloride, 5.0, agar, 15, casein, 10 and 0. 0015% (w/v) BCG- Bromocresol Green Dye) was prepared and streaked with bacterial isolate (Bacillus subtilis) and incubated at 37°C for 48 hrs [10]. It was observed that out of six strains (BS-1, BS-2, BS-3, BS-4, BS-5 and BS-6 isolated). A zone of proteolysis was detected on the casein agar plates of BS-5.
D) Optimization of media for maximum production of protease using different carbon sources-
The broth used for optimized production of protease enzyme consisted of varying carbon sources (Maltose, Glucose, Dextrose, Cellulose, Glycerol and Starch) 1% (w/v), casein 0.5%, yeast extract 0.55, KH2PO4 0.2%, Na2CO3 1%, MgSO4.7H2O 0.2%, and pH 8.0 at 140 rpm. [11-12] Optical density (OD) was taken using UV-visible spectroscopy at 660 nm at different time intervals and a graph was plotted (Table 1).
E) Calculation of dry weight of Bacillus subtilis (BS-5)-
Dry weight was calculated for each broth (having different carbon sources) containing the cultures after 96 hrs. 1 ml of cultures from each broth was transferred to centrifuge tubes of 1.5ml followed by centrifugation at 10,000 rpm for 15
minutes. The supernatant was discarded and the tubes containing the pellet were kept for air drying for overnight then the weight of cells was measured (Table 2).
RESULTS -
Table 1: Measurement of optical density of BS-5 using different carbon sources in broth
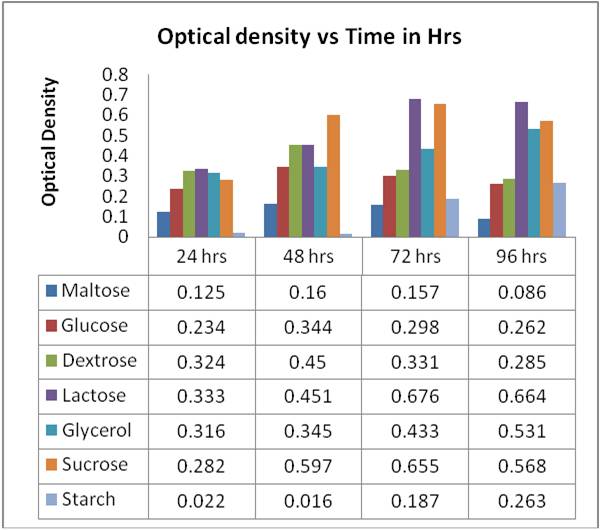
Table 2: Dry weight of Bacillus subtilis (BS-5)
| S. No. | Broth inoculated with BS-5 containing different Carbon sources | Dry Weight mg/ml |
| 1. | Maltose | 57 |
| 2. | Glucose | 77 |
| 3. | Dextrose | 89 |
| 4. | Lactose | 160 |
| 5. | Glycerol | 129.3 |
| 6. | Sucrose | 132.5 |
| 7. | Starch | 66 |
CONCLUSION- Characterization of the media is one of the key factors to maximize the yield of the product under study. When Optical Density of Bacillus subtilis was taken at 660nm the media (broth) containing lactose as carbon source was found to be maximum followed by sucrose. Dry weight was measured and calculated after 96 hrs and was found to be highest in lactose of 160 mg/ ml while it was 132.5 mg/ml in sucrose followed by glycerol with a yield of 129.3 mg/ml while in rest of the carbon source production was very less. In the broth the optical density and dry weight were highest in broth supplemented by lactose as carbon source as compare to other carbon sources supplemented broth and thus is ideal carbon source and can be used as it is the major by-product produces by cheese processing industry and is the major component of whey.
REFERENCES-
- Das G, Prasad MP, Isolation, purification & mass production of protease enzyme from Bacillus subtilis. Int. Research J of Microbio.2010; 1(2) 026.
- Degering C, Eggert T Puls M, Bongaerts J, Evers S, Maurer KH, Jaeger, KE, Optimization of Protease Secretion in Bacillus subtilis and Bacillus licheniformis by Screening of Homologous and Heterologous Signal Peptides. Applied and Environmental Microbiology American Society for Microbiology. 2010; 76(19): 6370–6376.
- Oyedele, Omowumi A, Ogunbanwo, Semuel, T. African Journal of Microbiology Research 2014; 8(18):1841-1849.
- Gupta R, Beg QK, Lorenz P, Bacterial alkaline proteases: molecular approaches and industrial applications. Appl. Microbiol. and Biotechnol. 2002; 59 (1): 15-32.
- Lowry OH, Rosebrough, NJ, Farr AL and Randall RJJ Protein measurement with the Folin phenol reagent. Biol. Chem. 1951; 193: 265.
- Akcan N, Uyar F, Production of extracellular alkaline protease from Bacillus subtilis RSKK96 with solid state fermentation. Eurasia J Biosci. 2011; 5:64-72.
- Sathiya G. Production of protease from Bacillus Subtilis And Its Application in Leather Making Process. International Journal of Research in Biotechnology and Biochemistry. 2013; 3(1): 7-10.
- Kumar R, Vats R, Protease Production by Bacillus subtilis Immobilized on Different Matrices. New York Science Journal. 2010; 3(7):20-25.
- Amin M, Rakhisi Z, Ahmady AZ, Isolation and Identification of Bacillus Species from Soil and Evaluation of Their Antibacterial Properties. Avicenna J Clin Microb Infec. 2015; 2(1):1-4.
- Vijayaraghavan P, Vincent, SGP. A simple method for the detection of protease activity on agar plates using bromocresol green dye. Journal of Biochemical Tech 2013; 4(3): 628-630.
- Geethanjali S, Subhash A, Optimization of protease production by Bacillus subtilus isolated from mid gut of fresh water fish Labeo Rohita. World journal of fish and marine science. 2011; 3(1):88-95.
- Nisha, NS, Divakaran, J. (2014).Optimization Parameters for Alkaline protease Production using Bacterial isolates from different coastal regions of Tamil Nadu, India. International Journal of Curr. Microbiol. App. Sci, 3(8): 500-505.
| Source of Financial Support: Nil Conflict of interest: Nil |