INTRODUCTION- Land constitutes a sensitive asset resource that acts as pivot in agriculture and other industries. Over depen-dence on land to solve the common challenges of man has overburdened land and exposed it to the effects of degradation which include but not limited to loss of biodiversity and soils nutrients, soil acidification, alkalinization, salinization and other soil management induced threats to agriculture; consequently, land is shaped and designed to suite the economic or political benefit man intends to derive.
This gives rise to land use which is the common series of operations carried out by man, with the intension to obtain products and benefits from land resources, hence inappropriate land use may aggravate the degradation of soil physicochemical and biological properties [1-3].
However, land use affects basic processes such as erosion, soil structure and aggregate stability, nutrients cycling, leaching, carbon sequestration and other physical and chemical processes [4].
The soil biological components are the active and pri-mary factors in the physical and chemical formations of soils [5], and soil organisms tend to maintain a fertile soil as a vital ecological service [6], with a concomitant decomposition and mineralization of plant materials and the subsequent release of nutrients for crop uptake [7-8]. Soils under similar climatic condition that are put into different land uses are likely to be different in their fertility status mainly as a result of the age and type of organic materials; the rate at which they drop and mineralize and the capacity of a given type of crop roots to hold nutrients in place and re-duce losses.
According to Oseni [9], forest soils are richer in chemical nutrients and microbial parameters than plantations or intensively cropped lands, while similar studies by Aisuene [10] revealed high total nitrogen and low available phosphorus, organic carbon and basic cations in oil palm plantations. However, the rate at which nutrients are depleted in different land use types shows that secondary forests < oil palm < arable cropping < building sites, with secondary forest being the most depleted [11].
Akamkpa Local Government Area (LGA) is richly blessed with diverse forest and mineral resources. Its highly dense tropical rain forest is a source of Gmelina, Teak, Opepe, Obeche, and numerous species of animals and insects. The area is a rich source of granitic materials and is known for its pure form of limestone in Nigeria. The exploitation of these resources, especially the solid minerals and forests for the purpose of development has severely affected and altered the natural ecosystem, affecting the soil nutrient reserve and having adverse effects on arable and tree crops in the area. This study is therefore imperative, as the physicochemical and microbial properties of the soils under different land use types will be assessed and the nutrient status compared with the view of identifying limiting nutrients. Ameliorative measures will also be suggested on limiting properties for improved productivity.
MATERIALS AND METHODS
Description of the Study Area- The study area is located in Akamkpa LGA (Longitude 50 00”, 50 57” N and Latitude 80 06, 90 00” E) in southern Cross River State, Southeast Nigeria. Akamkpa is bordered by Odukpani and Akpabuyo LGAs to the West and South respectively, Biase and Yakurr to the Northwest, Ikom and Etung LGA to the North and the Republic of Cameroun to the West. The area is characterized by distinct rainy and dry seasons with a short spell of Harmattan between November and February. It has an annual rainfall of 1300 – 3000 mm and annual temperature range of 21 – 300C and relative humidity of 75 – 80%. The study area is gentle to flat with well drained soils that are characterized by a mixture of stones in the soil surface. Akamkpa LGA is underlain by Basement Complex rocks and consist basically of upper older granites in all identified land use types, though parts of the area is underlain by Sedimentary materials of limestone, shale and sandstone intercalation.
Fig 1: Land Use Map of Akamkpa Local government Area
Field studies- Four land use types ranging from Rainforest and Gmelina plantation to Oil palm plantation as well as Rubber plantation were identified within Akamkpa LGA (Fig. 1). A sampling area measuring 30m x 30m was marked out in each land use type and soil samples collected with the aid of an auger from five points of the marked area (Fig. 2) at depths of 0–15cm and 15–30cm to represent top and subsoil respectively. At each depth, the five soil samples were bulked to form a composite sample. Eight representative composite soil samples were therefore collected from the four land use types in sampling bags, labeled and transported to the laboratory for physicochemical analyses. Soil samples for microbial analyses were collected aseptically in sterile poly bags, labelled and transported in ice parked coolers to the laboratory for analysis.
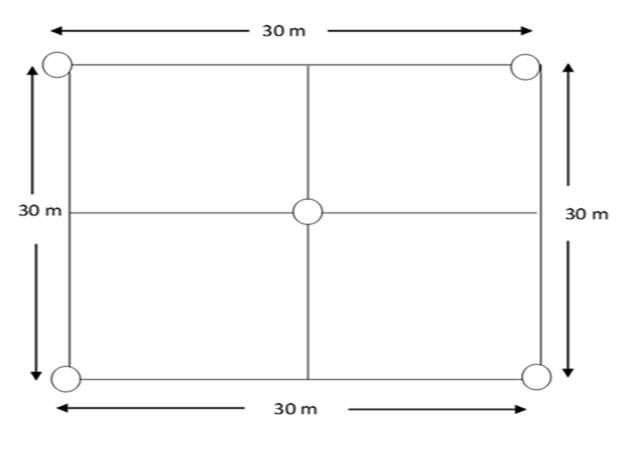
Fig. 2: Field Layout of the Sampling Area in each Land use type (Sampling points)
Microbial Analysis
Reagents used in the study- Soil Extract was prepared by suspending 1000 g of soil in 1 litter (1000 ml) of distilled water and stirred vigorously using stirring rod. The mixture was filtered with a Whatman No. 4 filter paper. The filtrate was sterilized by autoclaving at a temperature of 1210C and pressure of 1 b/sq. inch for 15 minutes. The extract was used in the preparation of agar for the estimation of total heterotrophic aerobic bacteria, purification and for stock culture. Malt extract agar was used for the isolation of fungi.
Enumeration of total heterotrophic Bacteria and Fungi- Soil samples were serially diluted in ten folds [17] at dilution factors of 10-6 and 10-3 for bacteria and fungi respectively. Total viable heterotrophic aerobic bacterial and fungal counts were determined using the pour plate technique. Molten soil extract agar and malt extract agar were poured into sterile Petri-dishes containing 1mL of the appropriate aliquot diluents for the isolation of total heterotrophic bacteria and fungi, however, plating was done in triplicates while observing all precautions. Colony counts were taken after incubating the plates at 300C for 24 and 48 hours for bacteria and fungi respectively. The bacterial isolates were sub cultured into nutrient agar slants which were then used for biochemical tests.
Characterization and Identification of isolates- Upon microscopic study of the bacterial isolates, characterization and identification was carried out at a magnification of X40 using an objective lens. Gram positive (+ve) organisms were seen as blue or violet colourations while red colours indicate gram negative (-ve) bacteria, however, spore formation, motility, oxidase and catalase production; citrate utilization, oxidative/fermentation (O/F) utilization of glucose; indole and coagulase production, starch hydrolysis, sugar fermentation, methyl red-Voges Proskaur reaction and urease production tests were also performed. The tests were performed by standard procedures [17- 24], while microbial identification was performed as outlined by Bergey [25]. Fungal isolates were examined macroscopically and microscopically using the needle mounts technique [26-27].
RESULTS AND DISCUSSION
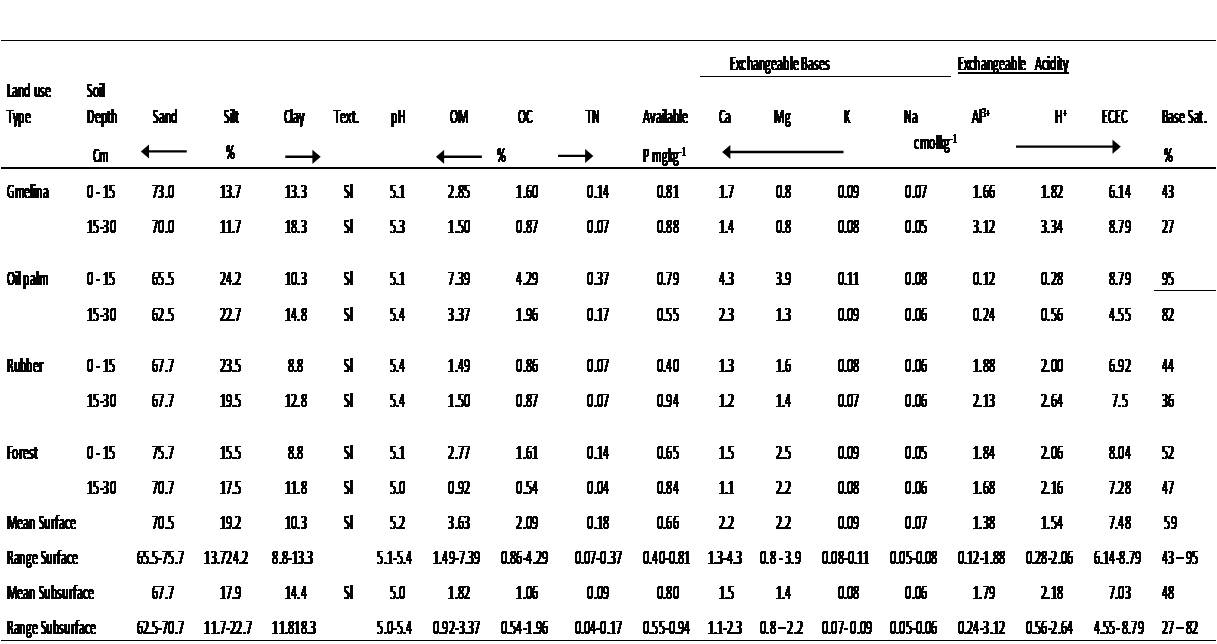
Physicochemical properties of the soils- The physicochemical properties of the soils are discussed with respect to Table 1. The particle size distribution was dominated by sand (Fig. 3), with values that ranged between 65.5 % in the Oil palm plantation and 75.7 % in the forest soils with a mean of 70.5 % in the surface soils. Silt fraction ranged between 13.7 % in the Gmelina plantation and 24.2 % in the Oil palm plantation while clay was averaged at 10.3 % in the entire surface soils (Fig. 3). The textural class was sandy loam irrespective of soil depth and land use type, an indication that the land use type had no effect on the textural class. Such sand dominated soils are likely to encourage water infiltration through the immediate soil surface, permit leaching of exchangeable bases and reduce the cation exchange surface on the mineral colloidal fraction.
The soil pH values ranged between 5.1 in Gmelina and Oil palm plantations and 5.4 in Rubber plantation with a mean of 5.2 in the surface soils. The soils are strongly acid [28] and are likely to encourage significant amounts of exchangeable Al3+ and H+ as to affect plant growth and encourage the solubility of Mn2+ and hinder plant root development.
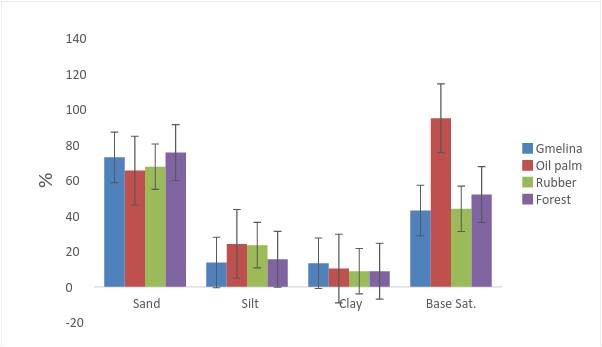
Fig. 3: Standard error bar charts showing physical properties for the soil surface
Irrespective of land use type, the entire soils are low in exchangeable Ca2+, K+ and Na+ but moderate in exchangeable Mg2+ [28]; however, soils of Oil palm plantation were strikingly higher in the basic cations, probably due to the high organic carbon/matter content and comparatively higher silt content which may have contributed to the soil’s cation exchange capacity. The fibrous and dense root system of Oil palm which concentrates in the soil surface, hold nutrients in place and decay to add organic materials to the soils which act as exchange cites for the basic cations. Exchangeable acidity was the sum of exchangeable Al3+and H+.
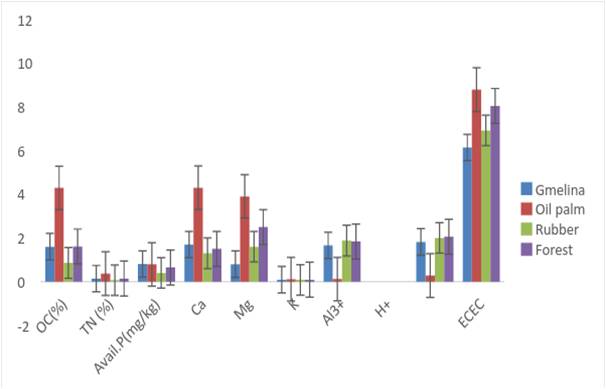
Fig. 4: Standard error bar charts showing chemical properties for the soil surface
Microbial properties of the soils- The microbial properties of the soils are discussed with respect to Tables 2 and 3. The probable bacterial isolates in the rhizosphere soils of Gmelina arborea plantation include Athrobacter spp, Agrobacterium spp, Bacillus spp, Micrococcus spp and Pseudomonas while the fungal isolates were Aspergillus niger, Paecelomyces spp, Pe-nicillium spp and Fusarium spp. Mean counts of 33.5 x106 and 29 x106 were obtained in the top and sub soil respectively for bacterial isolates and 20 x103 and 11 x103 x 103 in the top and sub soils for fungal isolates respectively. Furthermore, bacterial isolates identified in Elaeis guineensis plantation are Cellulosimicrobium spp, Enterobacter spp, Bacillus spp, Klebsiella spp, Pseudomonas spp, Enterococcus spp and Clostridium spp while Fusarium spp, Geotrichum spp, Verticillium spp, Penicillium spp, Trichoderma spp, Aspergillus spp, Mucor spp and Fusarium spp were the fungal isolates. Mean count of 48.2 x106 and 37.4 x106 were obtained in the top and sub soils respectively for bacterial isolates while fungal isolates had mean counts of 27 x103 and 20 x103 in the top and sub soils respectively. In the Rain Forest rhizosphere, bacterial isolates observed include Cladosporium spp, Penicillium spp, Verticillium spp, Aspergillus spp, Fusarium spp and Mucor spp while Cladosporium spp, Penicillium spp, Verticillium spp and Aspergillus spp as well as Fusarium spp and Mucor spp were obtained for fungal isolates. Bacterial isolates had a mean count of 34 x106 and 30.1 x106 in the top and sub soils while fungal isolates had means of 20 x103 and 17 x103in the top and sub soils respectively.
In the Hevea brasiliensis plantation, common bacterial isolates in the rhizosphere Actinomyces spp, Actinop-lanes spp, Arthrobacter spp, Streptomyces spp, Mi-cromonospora spp, and Gordona spp while Nocardia spp, Aspergillus spp, Mucor spp, Penicillium spp, Py-thium spp, Fusarium spp and Phytophthora spp were obtained as fungal isolates in the rhizosphere. Bacterial isolates recorded means of 33 x106 and 23 x106 in the top and sub soils respectively, while fungal isolates had means of 18 x103 and 11 x103 in the top and sub soils respectively. Elaeis guineensis plantation rec-orded the highest microbial count in the study area.
This is attributed to the high accumulation of litter materials from palm fronds, fruits and empty bunches droppings present at various stages of decomposition. Bacterial population was higher than the population of fungi, however, bacterial and fungal population were higher in the top soils than in sub soils across the plantations and the Rain Forest. This result is in line with the reports of [9, 25].
The different microbial communities isolated for the various land uses may be as a result of ecological alteration by human activities, changes in species composition of the plants, variation in the decomposition stages of litter materials and the availability of substrate for utilization by the microorganisms. This diversity in species of organisms at the different land uses is an indication that the land use types may have experienced altered biogeochemical cycling, due mainly to cultivation [30-31], field management [32], or contamination [33]. This is in agreement with the findings of Ajwa [34], that human activities have diverse effects on organic matter turnover and therefore, affect microbial activities because microbes are sensitive to disturbance.
Table 2: Mean bacterial Population and probable bacterial isolates in soils of the rainforest and mono plantations in Akampa LGA.
| Land Use | Plate count (cfu/g) (Top soil) | Plate count (cfu/g) (Sub soil) | Probable Bacterial Isolate |
|---|---|---|---|
| Gmelina arborea Plantation |
6 x 106 | 4 x 106 | Athrobacter spp |
| 4 x 106 | 12 x 106 | Agrobacterium spp | |
| 8 x 106 | 6 x 106 | Bacillus spp | |
| 6 x 106 | 4 x 106 | Micrococcus spp | |
| 5.5 x 106 | 3 x 106 | Pseudomonas spp | |
| Total | 33.5 x 106 | 29 x 106 | |
| Elaeis guineensis Plantation |
5 x 106 | 4 x 106 | Cellulosimicrobium spp |
| 4 x 106 | 3 x 106 | Enterobacter spp | |
| 16 x 106 | 13 x 106 | Bacillus spp | |
| 5 x 106 | 4 x 106 | Klebsiella spp | |
| 6 x 106 | 5.4 x 106 | Pseudomonas spp | |
| 5 x 106 | 3 x 106 | Enterococcus spp | |
| 7.2 x 106 | 5 x 106 | Clostridium spp | |
| Total | 48.2 x 106 | 37.4 x 106 | |
| Rain Forest | 1 x 106 | 2 x 106 | Athrobacter spp |
| 7 x 106 | 6.1 x 106 | Agromyces spp | |
| 5 x 106 | 3 x 106 | Bacillus spp | |
| 5x 106 | 5x 106 | Klebsiella spp | |
| 9x 106 | 6x 106 | Micrococcus spp | |
| 7x 106 | 8x 106 | Pseudomonas spp | |
| Total | 34 x 106 | 30.1 x 106 | |
| Hevea brasilinesis Plantation |
2 x 106 | 1 x 106 | Actinomyces spp |
| 4 x 106 | 3 x 106 | Actinoplanes spp | |
| 4 x 106 | 2 x 106 | Arthrobacter spp | |
| 5 x 106 | 3 x 106 | Streptomyces spp | |
| 4 x 106 | 3 x 106 | Micromonospora spp | |
| 2 x 106 | 1 x 106 | Gordona spp | |
| 12 x 106 | 10 x 106 | Nocardia spp | |
| Total | 33 x 106 | 23 x 106 |
Table 3: Mean fungal Population and probable fungal isolates from soils of the rainforest and mono plan-tations in Akampa LGA
| Land use type | Plate count (cfu/g) (Top soil) | Plate count (cfu/g) (Sub soil) | Probable Fungal Isolate |
|---|---|---|---|
| Plantation | 8 x 10-3 | 6 x 10-3 | Aspergillus niger |
| 3 x 10-3 | 2 x 10-3 | Paecelomyces spp | |
| 5 x 10-3 | 3 x 10-3 | Penicillium spp | |
| 4 x 10-3 | 2 x 10-3 | Fusarium spp | |
| Total | 20 x 10-3 | 11 x 10-3 | |
| Elaeis guineensis Plantation |
5 x 10-3 | 3 x 10-3 | Fusarium spp |
| 6 x 10-3 | 2 x 10-3 | Geotrichum spp | |
| 6 x 10-3 | 5 x 10-3 | Verticillium spp | |
| 4 x 10-3 | 5 x 10-3 | Penicillium spp | |
| 3 x 10-3 | 2 x 10-3 | Trichoderma spp | |
| 3 x 10-3 | 2 x 10-3 | Aspergillus spp | |
| 1 x 10-3 | Mucor spp | ||
| Total | 27 x 10-3 | 20 x 10-3 | |
| Rain Forest | 3 x 10-3 | 3 x 10-3 | Cladosporium spp |
| 6 x 10-3 | 4 x 10-3 | Penicillium spp | |
| 5 x 10-3 | 3 x 10-3 | Verticillium spp | |
| 2 x 10-3 | 2 x 10-3 | Aspergillus spp | |
| 5 x 10-3 | 2 x 10-3 | Fusarium spp | |
| 4 x 10-3 | 3 x 10-3 | Mucor spp | |
| Total | 20 x 10-3 | 17 x 10-3 | |
| Hevea brasilinesis Plantation |
3 x 10-3 | 2 x 10-3 | Aspergillus spp |
| 4 x 10-3 | 3 x 10-3 | Mucor spp | |
| 3 x 10-3 | 2 x 10-3 | Penicillium spp | |
| 4 x 10-3 | 2 x 10-3 | Pythium spp | |
| 2 x 10-3 | 1 x 10-3 | Fusarium spp | |
| 2 x 10-3 | 1 x 10-3 | Phytophthora spp | |
| Total | 18 x 10-3 | 11 x 10-3 |
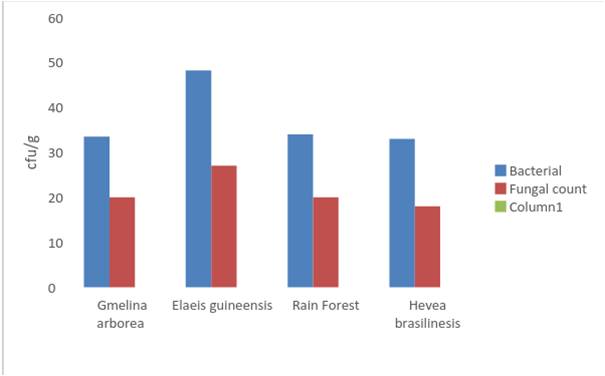
Fig. 5. Mean bacterial (x 106) and fungal (x103) Population from top soils of the rainforest and plantations in Akampa LGA
REFERENCES
- He, ZL; Alva, AK, Calvert, DV, Li, YC and Banks, DJ. 1999. Effects of N fertilizer of grape fruit trees on soil acidification and nutrient availability in a Riviera Fine Sand. Plant Soil 206: 11-19.
- Saikhe, H; Varadachari, C and Ghosh, K. 1998. Changes in carbon, N, and P levels due to deforestation and cultivation; A case study of Simplipal National Park, India; Plant Science, 198: 137-145.
- Singh, H; Sharma, KN and Arora, BS. 1995. Influence of continuous fertilization on maize system and the changes in soil fertility, Fertility Resource, 40: 7-19.
- Maddonni, GA, Urricariet, SS, Ghersa, CM and Lavado, RS. 1999. Assessing Soil fertility in the Rolling Pampa using Soil properties and maize characteristics, Journal of Agricultural Resource Science, 91: 280 – 286.
- Bardeth, R.D. 2005. The biology of soil: A community and Ecosystem approach, New York. Oxford University Press Inc.
- Baskin, Y 1997. The work of Nature; The scientific Community on problems of the environment (Scope), Washington D. C, Island Press.
- Bachamn, G; Zechmeister-Boltensteam, B; Pfefer, M and Hack, E. 2002. Microbial Nutrient Turnover in Soils of Natural forest communities.
- Pietikainen, J. 1999. Soil microbes in Boreal forest, humus after five. Finish Forest Research Institute, Research Paper, 75 (50): 78.
- Oseni, OA, Ekperigin, MM; Akindalunsi, AA and Oboh, G. 2007. Physicochemical and microbial properties of rainforest and plantation in Ondo State, Nigeria. African Journal of Agricultural Research, 2 (2): 605-609.
- Aisueni, N. O 1987. Impact of Oil palm (Eleasguineensis Jacq) cultivation on some chemical and microbiological properties of an Ultisol. Springer 1860 – 2000.
- Senjobi, BA and Ogunkunle, AO. 2011. Effects of different land use type and their Implications on land degradation and productivity in Ogun State, Nigeria. Journal of Agricultural Biotechnology and sustainable Development, 3 (1): 7-18.
- Gee, GW and Bauder, JW. 1986. Particle size analysis. Part I. Physical and Mineralogical methods, pp. 404-408. Madison, Wisconsin. U.S.A.
- International Institute of Tropical Agriculture (IITA). 1979. Methods of soil and plant analysis. Manual series of soil and plant analysis. IITA Ibadan.
- Bremner, JM 1965. Total Nitrogen. In C.A. Black (ed). Methods of Soil Analysis, Agronomy.9 American Society of Agronomy, Madison. WI pp. 1149-1178.
- Murphy, J and Riley, JP. (1962). A modified single solution method for determination of Phosphorus in natural waters. Analytica. Chemica. Acta. 27: 31-36.
- Thomas, GW. 1982. Exchangeable cations, Pp 159-165. In A. L. Page, A. Miller and D. R. Keeney (eds.) Methods of soil analysis part 2. (2nded). American Society of Agronomy and Soil Science Society of American. Madison, Wisconsin.
- Thomas, GW. 1996. Soil pH and Soil Acidity. In: D. L. Sparks (ed.) Methods of Soil Analysis Part 3–chemical methods, Soil Science Society of America Book Series 5, Madison, Wisconsin, pp 475-490.
- Zuberer, DA. 1994. Recovery and enumeration and viable bacteria. Method of soil analysis. Part 2 microbiological and bio-chemical properties. Soil Science Society of America book series. pp. 805 – 820.
- Alef, K. 1995. Nutrients, sterilization, aerobic and anaerobic culture techniques in applied soil microbiology and biochemistry. Academic Press Limited, 124(4): 110-130.
- Dennis, P. 1994. Flamentous fungi In: 571 Mikelson (ed.), Methods of soil analysis, part 2, microbiological and biochemical properties. Washington DC: Soil Science Society of America. Pp 365.373.
- Weaver, RA. and Peter, Graham, H. 1994. Method of soil analysis part 2 microbiological and bio-chemical properties. Soil Science Society of America book series. pp. 54-78.
- Harrigan, WF and Mc. Cance ME. 1990. Laboratory Methods in Food and Dairy Microbiology (8th Ed.) London: Academic Press.
- Cheesebrough, M. 2006. District laboratory practice in tropical countries. Part 2. Low Price Edition. Cambridge University Press, London, p 26.
- Agwung-Fobellah, D. and Kemajou, ST. 2007. Laboratory Microbiology and Activity manual. Ark of the Wisdom Publisher, Aba, Nigeria p 153.
- Ochei, JO and Kolhatkar, AA. 2008. Medical Laboratory Science: Theory and Practice, Tata. McGraw-Hill Publishing Company Limited, New York, pp 637-745.
- Bergey’s Manual of Determinative Bacteriology. 1994. 9th edition, Holt, J.D. (Ed.), Williams Wilkins CO. Baltimore, pp: 783.
- Barnett, HL and Hunter, BB. 1972. Illustrated genera of imperfecti fungi, 3rd edition, Burgess Publishing Company, Minnesota, U.S.A.
- Holland, MD, Barton, AD and Morph, ST. 1989. Land Evaluation for Agricultural Recommendation of Cross River National Park. Oban Division, prepared by Odwki, in collaboration with INNF.
- Adesemoye, AO, Opere, BO and Makinde, S.C.O. 2006. Microbial content of abattoir waste water and its contaminated soil in Lagos, Nigeria; Afr. J. Biotechnology, 5(20): 1963-1968.
- Anderson, TH & Domsch, KH. 1993. The metabolic quotient for CO2 (qCO2) as a specific activity parameter to assess the effects of environmental conditions, such as pH, on the microbial biomass of forest soils, Soil Biol. Biochemistry., 25: 393–395.
- Beyer, L; Blume, YP and Peters, M. 1991. Biological activity and organic matter transformations in typical soils of Schlesing-Holstein. Georderma, 49: 23-284.
- Perrott, KW, Sarathchandra, SU and Dow, BW. 1992. Seasonal and fertilizer effects on the organic cycle and microbial biomass in a hill country soil under pasture. Australian Journal of Soil Resources, 30:383-394.
- Chander, K and Brooke, PC. 1993. Residual effects of zinc, copper and nickel in sewage sludge on microbial biomass in a sandy loam. Soil Biology and Biochemistry, 25:1231-1239.
- Ajwa, HA, Dell, CJ, and Rice, CW. 1999. Changes in enzyme activities and microbial biomass of tall grass prairie soil as related to burning and nitrogen fertilization. Soil Biology Biochemistry 31:769-777.
| Source of Financial Support: Nil Conflict of interest: Nil |
|---|
| International Journal of Life-Sciences Scientific Research (IJLSSR) Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/ |
|---|