ABSTRACT- Thyroid disease commonly affects women of childbearing age and is the second most common
endocrinological disorder diagnosed in pregnancy after gestational diabetes. In normal gestation, the thyroid
gland adapts its structure and function to satisfy increasing functional demand. The marked physiological
changes that occur during normal pregnancy make it necessary to use specific reference ranges in interpretation
of thyroid function test. It is well documented that thyroid disorders are associated with maternal and fetal
complications during gestation, and its deleterious effects can also extend beyond pregnancy and delivery.
Available epidemiological data report widely varying prevalence rates of thyroid disorders during the antenatal
period. However, the need for universal thyroid screening remains controversial. Subclinical thyroid
dysfunction is very frequent but easily missed without specific screening programs. Furthermore, an appropriate
management is crucial to prevent adverse maternal and fetal outcomes. Despite the correlation between thyroid
function during pregnancy and maternal and fetal outcomes is a widely discussed issue, it remains important to
clarify several points regarding screening, diagnosis, and treatment of thyroid dysfunction in pregnant ladies. In
this article we try to discuss the physiological changes of the thyroid gland to meet the challenges of increased
metabolic demands during pregnancy and focusing on pathological function changes; we also try to summarize
the best way of screening, diagnosis and treatment of thyroid dysfunction during pregnancy to improve maternal
and fetal outcomes.
Key Words: Pregnancy, Thyroid gland, Hypothyroidism, Hyperthyroidism, Thyroid stimulating hormone
INTRODUCTION
Pregnancy has a profound impact on thyroid function. The
thyroid increases 10% in size during pregnancy in iodine
replete countries and by 20-40% in areas of iodine deficiency.
Production of Thyroxine (T4) and Triiodothyronine
(T3) increases by 50%, along with a 50% increase in the
iodine daily requirement. These physiological changes are
important to cope with an increase demand on thyroid
gland during pregnancy (1).
Thyroid disease is second to diabetes mellitus as the most
common endocrinopathy that occur in women during their
reproductive years. Symptoms of thyroid disease often
mimic common symptoms of pregnancy, making it
challenging to identify. Poorly controlled thyroid disease is
associated with adverse outcomes during pregnancy, and
treatment is an essential part of prenatal care to ensure
maternal and fetal well-being (2).
Thyroid function tests (TFT) interpretation in
pregnancy:
To meet the challenges of increased metabolic demands
during pregnancy, thyroid gland adapts several normal
physiological changes in hormone synthesis and
hypothalamic–pituitary-thyroid axis regulation.
Consequently, TFT results of healthy pregnant women differ
from those healthy non-pregnant women (Table 1) (3).
Table.1 Thyroid Function Test in Pregnancy
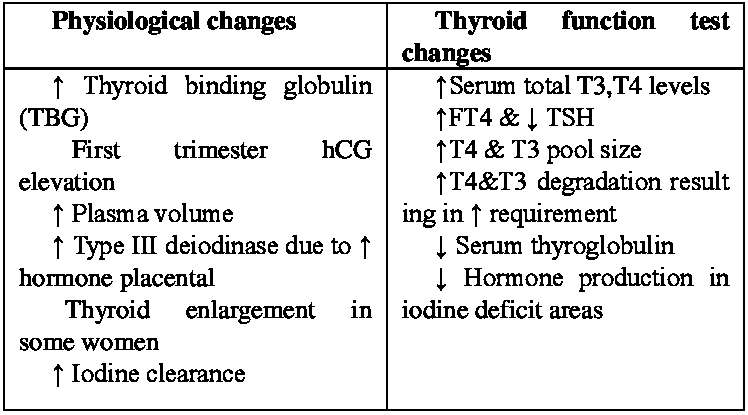
Changes in binding -serum protein level can influence measurement of FT4 that relay on estimates rather than direct measurements resulting inaccurate reported values (1,4).
Table. 2 Trimester – specific ranges for TSH and T4
| Test | Nonpregnant | 1st trimester | 2nd trimester | 3rd trimester |
| TSH mlU/L | 0.3-4.3 | 0.1-2.5 | 0.2-3 | 0.3-3 |
| FT4 ng/dL | 0.8-1.7 | 0.8-1.2 | 0.6-1 | 0.5-0.8 |
| TT4 mcg/dL | 5.4-11.7 | 6.5-11.1 | 7.5-10.3 | 6.3-9.7 |
Thyroid screening in pregnancy: Endocrine society recommends screening only pregnant women at high risk of thyroid diseases using serum TSH measurement, Table 3 (Endocrine Society 2012).
Table.3 Indications for thyroid screening in pregnancy
| -Current thyroid therapy -Goitre -Family history of autoimmune thyroid diseases -Personal history of: Autoimmune disorder High –dose neck radiation Previous delivery if infant with thyroid disease Postpartum thyroid dysfunction Therapy for hyperthyroidism Type 1 D.M |
Hypothyroidism: Maternal and Fetal Aspect: The incidence of hypothyroidism during pregnancy is estimated to be 2-3% for subclinical hypothyroidism, and 0.3-0.5% for overt hypothyroidism (5). It would be anticipated that such percentage would be higher in areas of iodine insufficiency. In iodine –sufficient region, the most common causes of hypothyroidism are autoimmune thyroiditis and iatrogenic hypothyroidism after treatment of hyperthyroidism (6) .Overt hypothyroidism (OH) is defined as TSH> 2.5 mU/L and low FT4, or TSH equal to 10 or above irrespective of FT4 levels.
On another hand, subclinical hypothyroidism (SCH) is defined as TSH between 2.5-10 mU/L and normal FT4 (1). Both overt hypothyroidism and subclinical hypothyroidism have adverse effects on the course of pregnancy and fetal development (7). On another hand, isolated hypothyroxinemia (low FT4 + normal TSH) has been found not to be associated with adverse perinatal outcomes Table 4 (8).
Table.4 Hypothyroidism and Pregnancy –Maternal and Fetal complications
| Condition | Preconception | Pregnancy | Postpartum |
| Overt Hypothyroidism (OH) | Decreased infertility, Increased abortion | Abortion, Preterm birth, Low birth weight, Fetal death, Gestational hypertension, Placenta Abruption, Preeclampsia, Fetal neurocognitive deficits |
Postpartum haemorrahe, Maternal thyroid dysfunction |
| Subclinical Hypothyroidism (SCH) | Similar to over hypothyroidism but less documentation exists. |
A large amount of retrospective and case-controlled studies confirms the detrimental effect of overt hypothyroidismon pregnancy and fetal health. Moreover, many data provide circumstantial evidence supporting an increased risk of adverse outcomes from maternal subclinical hypothyroidism. Table 5 summarized some of these trial`s results.
Table. 5 Summary of some trials demonstrated adverse effects of overt hypothyroidism (OH) & subclinical hypothyroidism (SCH) on pregnancy outcomes
| Albalovich et al (9) | Leung et al (10) | Negro et al (11) | Benhadi et al (12) | Casey et al (13) | Ashoor et al (14) | |
| Type | OH | OH | SCH | SCH | SCH | SCH |
| Results | 60% risk of fetal loss in untreated overt hypothyroidism in pregnant women | 22% risk of gestational hypertension in women with overt hypothyroidism | Significant reduction in a combined end points of pregnan cy complications in treated women with TSH >2.5Mu/l in both TPOAb positive or negative SCH | Increased risk of child loss with higher TSH | 2-3 folds increased risk of pregnancy– related complications in untreated women | Increased risk of abortion and felt death in untreated women with TSH above 97.5th percentile and FT4 below 2.5th percentile |
Either to due iodine deficiency or autoimmune thyroid disease, a reduction of circulating of maternal thyroxine has been shown to result in lower I.Q in infants in retrospective (15) and prospective (16) studies. However, results from Controlled Antenatal Thyroid Screening (CATS) study suggest a caution and a degree of uncertainty relating to this approach (17). Results of the second wave of the controlled Antenatal Thyroid Screening (CATS II), which is an extension study, are still pending (18).
L-T4 therapy is the mainstay for treatment for maternal hypothyroidism (2). The aim of treatment to normalize maternal serum TSH values within trimester–specific pregnancy reference range Table 1. It is strongly recommended not to use of T/T3 combination or desiccated thyroid during pregnancy (1).
All overt hypothyroidism cases should be treated during pregnancy. Women who have a TPOAb positive subclinical hypothyroidism, treatment should be considered. However; there is insufficient evidence to recommend for or against universal treatment of TPOAb negative women with subclinical hypothyroidism (1).
When a woman with hypothyroidism gets pregnant, the preconception L-T4 dose should be increased , and may require up to a 30-50% increment, as soon as possible to ensure that TSH <2.5 mU/L (19)
Serum TSH and FT4 should be measured every 4-6 weeks until 20 weeks` gestation and until the patient on stable medication dose; it should be measured again at 24-28 weeks and 32-34 weeks` gestation (20).
After delivery, L-T4 should be decreased to the prepregnancy dosage over a four– week period, and further adjustment should be guided by TSH levels 4-6 weeks after delivery (1).
Hyperthyroidism and pregnancy: In general, hyperthyroidism is less common than hypothyroidism, with an approximate incidence during pregnancy of 0.2% (5). In the first trimester of normal pregnancies, TSH levels usually are suppressed due to a stimulatory effect of hCG on the TSH receptors (21); therefore a suppressed TSH should be evaluated in conjunction with serum FT4. The diagnosis of hyperthyroidism is confirmed by suppressor undetectable TSH and an elevated FT4 (1). Grave`s disease accounts for 90% of hyperthyroidism (22) along with other causes Table 6.
| Table. 6 Etiology of hyperthyroidism in pregnancy |
| Nodular goitre or Toxic solitary adenoma Gestational trophoblastic disease Viral thyroiditis Pituitary tumors–Secondary Hyperthyroidism Ovarian tumors |
Transient hyperthyroidism may also be associated with hyperemesis gravidarum and gestational hyperthyroidism (23). No prior history of thyroid disease, absence of eye sings or goitre favour the diagnosis of gestational hyperthyroidism rather than Grave`sdisease. TRAb level can be diagnostically useful in doubtful cases (24). Supportive therapy is an appropriate management in gestational hyperthyroidism (25).
The natural history of hyperthyroid disorders varies with the underlying aetiology. Grave`s disease is typically characterized by an initial exacerbation in the first trimester thought to be caused by the additive stimulatory effect of hCG on thyroid gland. Symptoms usually improve during the second trimester, only to worsen again in postpartum period (5).
Overt hyperthyroidism that is inadequately treated is associated with a detrimental effect on maternal and neonatal outcomes (26) Table 7.
According to study by Casey BM et al 2005 (13), a more than 25000 women with subclinical hyperthyroidism have been studied. It has been shown no increment in adverse pregnancy outcomes; therefore, treatment is not recommended in these cases.
Table.7 Hyperthyroidism and Pregnancy-Maternal and Fetal complications
| Condition | Preconception | Pregnancy | Postpartum |
| Overt Hyperthyroidism | Congenital malformation | Fetal: goitre, IUGR, small for gestational age, stillbirth, thyroid dysfunction Maternal: Preterm delivery, preeclampsia, placental abruption, thyroid storm, congestive heart failure (27) | Neonatal thyroid dysfuction, neonatal goitre |
| Subclinical hyperthyroidism | -------- | none | ---------- |
Hyperthyroidism during pregnancy is treated with anti-thyroid drugs (28). Because Methimazol is associated with birth defects such as aplasia cutis and choanal or oesophageal atresia (29). Propylthiouracil is a preferred drug during first trimester (30). However, it is recommended to switch to Methimazol after the first trimester because of the risk of hepatotoxicity associated with Propylthiouracil use (31).
The essential practical aspect of Grave`s hyperthyroidism management during pregnancy should include:
Discuss treatment-related issues with patients such as effect on patient, effect on fetus, and breast feeding.
Start propylthiouracil –use for the first trimester.
Render patient euthyroid-continue with low dose carbomazol or methimazole to ensure a serum FT4 level at or moderately above reference range (1).
Serum FT4 and TSH levels should be monitored every 2-6 weeks.
Women with active Grave`s disease, a history of Grave`s disease treated with radioactive iodine or thyroidectomy, or a history of delivering an infant with hyperthyroidism should checked for TRAb at 24-28 weeks gestation (32).
In women at high risk , including those with uncontrolled hyperthyroidism or high TSH receptor antibodies (TRAbs) titre, fetal surveillance and ultrasonography should be performed monthly after week 20 gestation to detect evidence of fetal thyroid dysfunction( e.g goitre, hydrops, growth restriction , heart failure (33).
Inform paediatrician.
Check infant for thyroid dysfunction if indicated.
Review postpartum-check for exacerbation.
In the same line, ablation therapy with RAI is contraindicated in pregnancy and lactation. Thyroidectomy is rarely considered in second trimester in cases with ATDs side effects, requiring a higher ATDs dose, or non-complaint with drug therapy (1).
Postpartum Thyroid Dysfunction: Postpartum thyroiditis is the most common cause of postpartum thyroid dysfunction, which affects 1.1% -21.1% of women (34). Patients with Type 1 diabetes and women with high TPOAb or TgAb titres are at increased risk of postpartum thyroiditis (35). The clinical course of postpartum thyroiditis varies (1) as approximately 25% of patients present with hyperthyroidism, followed by hypothyroidism, and then recovery.
Furthermore, 43% of patients with postpartum thyroiditis present with symptoms of hypothyroidism and 32% may present with hyperthyroidism (1).
Practically speaking, treatment of hyperthyroidism is generally symptomatic using B-blockers and no role for ATD. In contrast, postpartum hypothyroidism should be treated with levethyroxine in symptomatic (36). Women with history of PT are at increased risk of permanent hypothyroidism and should be screened annually then after (37).
CONCLUSIONS
There is a consensus that pregnancy imposes a stress on the thyroid which is greater in iodine –deficit areas. All women with thyroid disorders should counsel about the importance of achieving euthyroidism before conception to avoid poor outcomes. Hypothyroidism is more common than hyperthyroidism, and both required appropriate management to improve maternal and fetal outcomes.
REFERENCES
- Stagnaro-Green A, Abalovich M, Alexander E, et al 2011. American Thyroid Association Taskforce on Thyroid Disease during Pregnancy and Postpartum. Guidelines of the American Thyroid Association for the diagnosis and management of thyroid disease during pregnancy and postpartum, Thyroid 21(10):1081–1125,
- De Groot L, Abalovich M, Alexander EK, et al 2012. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab, 97(8): 2543–2565.
- Brent GA 1997. Maternal thyroid function: interpretation of thyroid function tests in pregnancy. Clin Obstet Gynecol, 40:3-15.
- Abbassi-Ghanavati M, Greer LG, Cunningham FG 2009. Pregnancy and laboratory studies: a reference table for clinicians]. Obstet Gynecol. 114(6):1326–1331.
- Neale DM, Cootauco AC, Burrow G 2007 Thyroid disease in pregnancy. Clin Perinatol, 34(4):543–557.
- Allan WC, Haddow JE, Palomaki GE, Williams JR, Mitchell ML, Hermos RJ, Faix JD, Klein RZ 2000 Maternal thyroid deficiency and pregnancy complications: implications for population screening.J Med Screen, 7:127–130.
- Wasserstrum N, Anania CA 1995 Perinatal consequences of maternal hypothyroidism in early pregnancy and inadequate replacement. Clin Endocrinol (Oxf), 42:353-358.
- Casey BM, Dashe JS, Spong CY, et al 2007. Perinatal significance of isolated maternal hypothyroxinemia identified in the first half of pregnancy. Obstet Gynecol, 109:1129-35.
- Abalovich M, Gutierrez S, Alcaraz G, Maccallini G, Garcia A, Levalle O 2002 Overt and subclinical hypothyroidism complicating pregnancy. Thyroid, 12:63–68.
- Leung AS, Millar LK, Koonings PP, Montoro M, Mestman JH 1993 Perinatal outcome in hypothyroid pregnancies. Obstet Gynecol, 81:349–353.
- Negro R, Schwartz A, Gismondi R, Tinelli A, Mangieri T, Stagnaro-Green A 2010 Increased pregnancy loss rate in thyroid antibody negative women with TSH levels between 2.5 and 5.0 in the first trimester of pregnancy. J Clin Endocrinol Metab, 95:E44–8.
- Benhadi N, Wiersinga WM, Reitsma JB, Vrijkotte TG, Bonsel GJ 2009 Higher maternal TSH levels in pregnancy are associated with increased risk for miscarriage, fetal or neonatal death. Eur J Endocrinol, 160: 985–991.
- Casey BM, Dashe JS, Wells CE, McIntire DD, Byrd W, Leveno KJ, Cunningham FG 2005 Subclinical hypothyroidism and pregnancy outcomes. Obstet Gynecol, 105:239–245.
- Ashoor G, Maiz N, Rotas M, Jawdat F, Nicolaides KH 2010 Maternal thyroid function at 11 to 13 weeks of gestation and subsequent fetal death. Thyroid, 20:989–993.
- Haddow JE, Palomaki GE, Allan WC, et al 1999. Maternal thyroid deficiency during pregnancy and subsequent neuropsychological development of the child. N Engl J Med, 341:549-5.
- Pop VJ, Brouwers EP, Vader HL, et al 2003. Maternal hypothyroxinaemia during early pregnancy and subsequent child development: a 3-year follow-up study. Clin Endocrinol, 59: 282-8.
- Lazarus JH, Bestwick JP, Channon S, Paradice R, Maina A, Rees R, Chiusano E, John R, Guaraldo V, George LM, Perona M, Dall'Amico D, Parkes AB,Joomun M, Wald NJ 2012. Antenatal thyroid screening and childhood cognitive function. N Engl J Med, 366(6): 493-501.
- Hales C, Channon S, Taylor PN, Draman MS, Muller I, Lazarus J, Paradice R, Rees A, Shillabeer D, Gregory JW, Dayan CM, Ludgate M 2014. The second wave of the Controlled Antenatal Thyroid Screening (CATS II) study: the cognitive assessment protocol. BMC Endocr Disord, 14:95.
- Abalovich M, Alcaraz G, Kleiman-Rubinsztein J, Pavlove MM, Cornelio C, Levalle O, Gutierrez S 2010. The relationship of preconception thyrotropin levels to requirements for increasing the levothyroxine dose during pregnancy in women with primary hypothyroidism. Thyroid, 20:1175–1178.
- ]Abalovich M, Amino N, Barbour LA, et al 2007. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab, 92:S1-47.
- Glinoer D, Spencer CA 2010 Serum TSH determinations in pregnancy: how, when and why? Nat Rev Endocrinol, 6:526– 529.
- Marx H, Amin P, Lazarus JH 2008. Hyperthyroidism and pregnancy. BMJ, 22:663-7.
- Goodwin TM, Montoro M, Mestman JH 1992. Transient hyperthyroidism and hyperemesis gravidarum: clinical aspects. Am J Obstet Gynecol, 167: 648–652.
- Tan JY, Loh KC, Yeo GS, Chee YC 2002. Transient hyperthyroidism of hyperemesis gravidarum. BJOG, 109:683–688.
- Niebyl JR 2010. Clinical practice Nausea and vomiting in pregnancy. N Engl J Med, 363:1544–1550.
- Millar LK, Wing DA, Leung AS, Koonings PP, Montoro MN, Mestman JH 1994. Low birth weight and preeclampsia in pregnancies complicated by hyperthyroidism. Obstet Gynecol, 84:946–949.
- Sheffield JS, Cunningham FG 2004. Thyrotoxicosis and heart failure that complicate pregnancy. Am J Obstet Gynecol, 190:211–217.
- Mandel SJ, Cooper DS 2001. The use of antithyroid drugs in pregnancy and lactation. J Clin Endocrinol Metab, 86:2354– 2359.
- Di Gianantonio E, Schaefer C, Mastroiacovo PP, et al 2001. Adverse effects of prenatal methimazole exposure. Teratology, 64(5):262–266.
- Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, McDougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN 2009.The role of propylthiouracil in the management of Graves' disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid, 19:673–674.
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P 2004. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl, 10:1018–1023.
- Mitsuda N, Tamaki H, Amino N, Hosono T, Miyai K, Tanizawa O 1992. Risk factors for developmental disorders in infants born to women with Graves disease. Obstet Gynecol, 80:359–364.
- ACOG practice bulletin 2000. Antepartum fetal surveillance. Clinical practice management guidelines for obstetriciangynecologists. Int J Gynaecol Obstet, 68(2):175–185.
- Muller AF, Drexhage HA, Berghout A 2001. Postpartum thyroiditis and autoimmune thyroiditis in women of childbearing age: recent insights and consequences for antenatal and postnatal care. Endocr Rev 22(5): 605–630.
- Mamede da Costa S, Sieiro Netto L, Coeli CM, Buescu A, Vaisman M 2007. Value of combined clinical information and thyroid peroxidase antibodies in pregnancy for the prediction of postpartum thyroid dysfunction. Am J Reprod Immunol, 58(4): 344–349.
- Yassa L, Marqusee E, Fawcett R, Alexander EK 2010 Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J Clin Endocrinol Metab, 95(7): 3234– 3241.
- Azizi F 2005. The occurrence of permanent thyroid failure in patients with subclinical postpartum thyroiditis. Eur J Endocrinol, 153(3):367–371.
| International Journal of Life-Sciences Scientific Research (IJLSSR)
Open Access Policy
Authors/Contributors are responsible for originality, contents, correct
references, and ethical issues.
IJLSSR publishes all articles under Creative Commons
Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |