ABSTRACT- Striga is a major constraint affecting sorghum, maize, other cereal crops, sugar cane and legume crops
production in sub Saharan Africa. Striga may result in complete crop loss under the worst of conditions. Prodigious seed
production, prolonged viability of the seeds and the subterranean nature of the early stages of parasitism make the control
of the parasite by conventional methods difficult if not impossible. The increasing incidence of Striga has been attributed
to poor soil fertility and structure, low soil moisture, intensification of land use through continuous cultivation and an
expansion of cereal production. Many potentially successful approaches developed to control this weed include using
resistant/tolerant varieties, sowing clean seeds that are not contaminated with Striga seeds, rotating cereal hosts with trap
crops that induce abortive germination of Striga seeds, intercropping, applying organic and inorganic soil amendments
such as fertilizer or manure, fumigating soil with ethylene, applying post emergence herbicides, push-pull technology and
using biological control agents. Based on some studies, the interaction of tied-ridging with N fertilizer and resistant
varieties; cereal-legume intercropping and its interaction with N fertilizer revealed low Striga infestation. No single
management option has been found effective across locations and time. Hence, an integrated Striga management
approach, currently, offers the best possibility for reducing impact at the farm level.
Key Words- Intercropping, Integrated pest management, Fertilizer, Management options, Striga
INTRODUCTION
Agriculture remains the main source of food and provides
the primary source of livelihood for 36% of the world’s
total workforce [1]. In Asia and the Pacific, 40 to 50% of
the workforce derives its livelihood from agriculture, while
in sub-Saharan Africa (SSA) two-thirds of the working
population still makes their living from agriculture.
In Ethiopia, about 85% of the population depends on
agriculture out of which over 90% still rely on rain-fed
agriculture for their livelihood [2].
The majority of the population in the Arid and Semi-arid
areas depend on agriculture and pastoralism for
subsistence. These activities face many constraints due to
predominance of erratic rainfall patterns, torrential rainfall
which is majority lost to run-off, high rate of evapotranspiration
further reducing yields, weeds growing more
vigorously than cultivated crops and competing for scarce
reserves of moisture, low organic matter levels and high
variables responses to fertilizers [3].
Among the major pests of agricultural crops, weeds alone
caused severe yield losses ranging from as low as 10% to
as high as 98% of total crop failure in the dry land regions.
It should be emphasized that yield losses caused by weeds
could vary from crop to crop and from region to region for
the same crops, in response to many factors that include:
weed pressure, availability of weed control technology, cost
of weed control and level of management practices [4].
From the parasitic weeds, Striga spp. are fairly wide spread
in semi-arid regions crops including certain legumes,
maize, pearl millet, sorghum, other cereal crops and sugar
cane production. Small holder farmers are the most affected
by the Striga problem because they have limited ways and
means of controlling it. The increasing incidence of Striga
has been attributed to poor soil fertility and structure, moisture
stress, intensification of land use through continuous
cultivation and an expansion of cereal production
[5-6]. Most Striga infested areas are characterized by
agricultural production systems exhibiting low
productivity.
DISTRIBUTION AND HOST RANGE OF
STRIGA:
Striga has been given the common name of "witchweed"
because it attaches itself to the roots of the host plant thus
depriving it (the host) of water and nutrients. Striga spp.
(witch weeds) belongs to the family Orobanchaceae [7].
Economically important Striga species are reported from
more than 50 countries, especially from East and West
Africa and Asia [8]. S. hermonthica is common throughout
northern tropical Africa and extends from Ethiopia and
Sudan to West Africa. It also extends from the western
Arabian region southwards into Angola and Namibia [6].
S. asiatica has a wider distribution and is found throughout
semi-arid areas of tropical and subtropical Africa, Asia and
Australia [6]. Nigeria, Sudan, Ethiopia, Mali and Burkina
Faso are heavily affected counties in Africa [9].
The host range is almost wide and besides the cultivated
cereals, it attacks many of the wild grasses. The traditional
crops in the African savanna attacked by the parasite are
sorghum (Sorghum bicolor L., maize (Zea mays L.), pearl
millet (Pennisetum glaucum L.), and sugarcane (Saccharum
officinarum L.) and rice (Oriza sativa L.) [10].
STRIGA BIOLOGY:
Striga plants have green opposite leaves, bright irregular
flowers with corolla tube slightly bent at the middle. The
flowers are pink, red, white or yellow. There is a considerable
variation in flower color. The plant is characterized by
herbaceous habit, small seeds and parasitism. The seeds of
S. hermonthica are extremely small, about 0.2 X 0.3mm,
weighing about 0.7µg. They are generally dispersed by
water, wind, cattle, and man .The number of seeds per
capsule ranges from 700 – 1800 depending on the species.
The seeds can remain viable in the field for as long as
14-20 years .The minimal length of the life cycle of the
parasite, from germination to seed production comprises an
average of 4 months [10].
Since Striga is a parasitic weed the seedlings cannot sustain
themselves on their own resources for particular long after
germination. Therefore, they need to find a host root
shortly after germination and the germination needs to be
perfectly timed with the presence of a host root. Exogenous
germination stimulants called strigolactones are produced
by the host’s root and also by some non-host (usually
referred to as trap crops) roots (Gossypium sp.). They are
plant hormones which inhibit shoot branching [11] but also
signals to seeds of parasitic weeds such as Striga to start
germinate. Strigolactones are also involved in other
physiological processes such as abiotic response and the
regulation of the plants structure is also regulated by
strigolactones. Strigol, a synthetic compound belonging to
the strigolactones, was first isolated from cotton
(Gossypium sp.) and is used as a germination trigger for
Striga [12]. When the seed have been germinated the
seedling can live for 3 to 7 days without a host. After that it
will die if it is not attached to a root and there has been able
to create a parasitic link to that particular root. The seedling
finds its way to the host root by chemical signals and then
creates a xylem-to-xylem connection between the seedling
and the root. However, the seedlings cannot be at a greater
distance from the root than 2 to 3 mm to find its way there.
When the seedlings have attached to the root it grows
underground for 4-7 weeks before they emerge and are
actually seen in the field. One plant can host many Striga
plants and Striga affects the plant mostly before its
emergence. The symptoms are however hard to distinguish
from symptoms caused by drought, lack of nutrients and
other diseases [10].
Subsequent to germination, which occurs in close
proximity of the host roots, a haustorium (organ of
attachment and a physiological bridge between the host and
the parasite) is produced on perception of a host-derived
chemical signal [13]. Haustorium initiation, which
represents the switch from the vegetative to the parasitic
mode of life, occurs on or near the host. The haustorium
attaches, penetrates the host root and establishes connection
with the host xylem. Following attachment, the parasite
remains subterranean for six to eight weeks [13]. During
this period, the parasite is completely dependent on its host
and is most damaging. Generally, the below ground and
above ground development of Striga is shown in the life
cycle of Striga (Figure 1.).
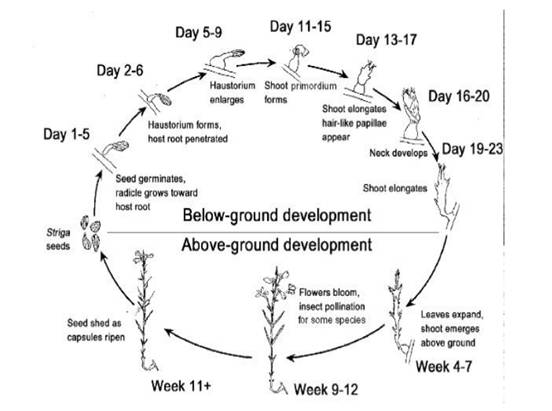
Fig 1 : General life cycle of Striga species
Source: Striga Research Methods [14].
ECONOMIC IMPORTANCE OF STRIGA: Although there are more than 35 species, only three species are recognized as economically important [15]. S. hermonthica (Del.) Benth and S. asiatica (L.) Kuntze are the two most widespread and the most economically significant species that parasitize on sorghum (Sorghum bicolor L. Moench), pearl millet (Pennisetumglaucum L.), maize (Zea mays L.) and rice (Oryza sativa L.), whereas S. gesnerioides (Willd.) Vatke attacks crops such as cowpea (Vignaunguiculata L. Walp.) and peanut (Arachishypogaea L.) [16]. Of these speciesStriga hermonthica is the most serious biotic problem to cereal production [10]. S. hermonthica is a debilitating root parasite. It causes damage in two ways, first by competition for carbon and nutrients and second through physiological interactions, and metabolic processes the bulk of which is unknown [17].
The effect of Striga damage on crops is a reduction in yield. The extent of yield loss is related to the incidence and severity of attack, the host’s susceptibility to Striga, environmental factors (edaphic and climatic) and the management level at which the crop is produced [18]. Its effects on crops range from stunted growth, through wilting, yellowing, and scorching of leaves, to lowered yields and death of many affected plants. A report by [9] indicated that annual sorghum losses attributed to Striga in SSA are estimated at 22-27% and specifically at 25% in Ethiopia, 35% in Nigeria, and 40% in Mali. In terms of monetary value, the annual cereal losses due to Striga are estimated at US$7 billion in SSA. In Ethiopia, Mali and Nigeria, the annual losses are estimated at US$75 million, US$87million and US$1.2 billion respectively [9]. In Sudan, more than 500,000 hectares under rain fed cultivation are heavily infested by Striga, which commonly results in yield losses of 70–100% and thus severe Striga infestation can result in complete crop failure [10].
MANAGEMENT AND CONTROL OPTIONS OF STRIGA: [19] opined that Striga is a particular problem in areas with low moisture and where soil fertility is being eroded through increased population pressure, decreased use of fallow and minimal use of organic or inorganic fertilizer. Most importantly, it mostly affects the livelihoods of poor subsistence farmers in cereal-based agricultural systems in Africa. Prodigious seed production, prolonged viability of the seeds and the subterranean nature of the early stages of parasitism make the control of the parasite by conventional methods difficult if not impossible [10]. Several measures have been tried and adopted for control of Striga. Many potentially successful approaches developed to control this weed include using resistant/tolerant varieties, sowing clean seeds that are not contaminated with Striga seeds, rotating cereal hosts with trap crops that induce abortive germination of Striga seeds, intercropping, applying organic and inorganic soil amendments such as fertilizer or manure fumigating soil with ethylene, hoeing and hand pulling of emerged Striga, applying post emergence herbicides, push-pull technology and using biological control agents [10]. Generally, the approaches can be grouped in to four independent Striga control options, namely cultural, chemical, genetic, and biological.
Cultural management practices: Effective control of Striga has been difficult to achieve through conventional hand or mechanical weeding as the parasite exerts its greatest damage bewitching the crop before its emergence above ground, and providing evidence for host plant infection. Many of the traditional control methods, including crop rotation, soil fertility, trap and catch cropping, intercropping, hand-pulling and fertilization are still in vogue [10]. Still these practices are not adopted by farmers. Because they are perceived by poor farmers as unaffordable or uneconomical, labor intensive, impractical, or not congruent with their other farm operations. A lot of studies have been reported mainly on the effect of intercropping and fertilizer against Striga as follows.
A. Intercropping practice on Striga management: Weed control is an important aspect in intercropping because chemical control is difficult once the crops have emerged. A study by [20] showed that intercropping maize with legumes considerably reduced weed density in the intercrop compared with maize pure stand due to decrease in the available light for weeds in the maize-legume intercrops, which led to a reduction of weed density and weed dry matter yield compared with sole crops. Similarly, [21] demonstrated that intercropping maize or sorghum with the fodder leguminous Desmodium uncinatum (Jacq.) DC. and D. intortum (Mill.) Urb, significantly reduced S. hermonthica infestation and increased grain yield. Similar studies in Kenya indicate that intercropping with cowpeas between the rows of maize significantly reduced Striga numbers when compared to within the maize rows [22]. Moreover, finger millet (Eleusinecoracana) intercropped with green leaf desmodium (Desmodium intortum) reduced Striga hermonthica counts in the intercrops than in the monocrops [23]. [24] also reported related findings on sorghumcowpea intercropping where Striga emergence was lower under intercrops than sole crops. Generally, various studies have shown that intercropping cereals, mainly with legumes such as cowpea (Vignaunguiculata), peanut (Arachis hypogaea) and green gram (Vigna radiate) can reduce the number of Striga plants [25]. Potentially, they might be acting as traps crops, stimulating suicidal Striga germination or the microclimate under the crop canopy may be altered and interfere with Striga germination and development [26]. It is also hypothesized that nitrogen fixed by the legumes might interact with Striga growth, as increasing the amount of available nitrogen can reduce Striga densities [27].
B. Fertilizer application on Striga management: AsStriga is more favor in less fertile soil, a system that would improve soil fertility to increase yield as well as reduce Striga infestation will be also of double advantage. Good soil management practices involving the use of crop residues and organic manure have been effective control measure against Striga. [28] observed that Striga infestation decreased with increasing organic matter of the soil and that organic matter content seemed to be the most important factor which preserved the soil fertility. Since soil microbial biomass flourishes better in a medium rich in organic matter, organic or inorganic soil amendments may increase soil suppressiveness to Striga spp. and also improve soil conditions to increase yield of subsequent cereal. Different research findings were reported by authors. According to [29], 55-82% reduction in number and weight of S. hermonthica recorded due to application of N using urea in Niger. [30] also reported that N fertilizers altered assimilate partitioning in favour of the ear and increased maize grain yield and reduced Striga count by 64%. Similarly, the study of [31] conducted in North east Nigeria showed a reduction in Striga infestation and damage with the application of N fertilizer on maize varieties. Striga infestation was significantly reduced at 120 kg N ha-1 in the early variety and 60 and 120 kg N ha-1 in late varieties. [32] noted that, the nitrogenous compound fertilizer which contains urea considerably suppressed germination of S. hermonthica when applied during conditioning.
The germination of S. hermonthica seed is associated with the secretion of germination stimulants by host plants. The secretion ultimately depends upon the nutrient status of the soil [33]. It has been demonstrated that under N and P deficiency, host plants secrete high amounts of germination stimulants into the rhizosphere, while supply of sufficient N and P reduces this secretion [34, 35]. Research studies showed that the effect of N was less pronounced than the effect of P on strigolactones secretion. As DAP fertilizer contains 18% N and 46% P2O5, high availability of P in DAP might lead to less production of strigolactones. However, direct suppressing effect of N on Striga spp. cannot be neglected [36].
The high and increasing cost of mineral fertilizers and low purchasing power of small scale farmers have necessitated investigating the efficacy of fertilizer application at low to very low levels. The use of very low doses of mineral fertilizers and their placement near the planting hole, a technology termed ‘microdosing’, have been shown to reduce application rates and thus cost of fertilizer per surface area, while still improving crop yields [37]. Microdosing of DAP may prove to be an efficient and cost effective option to reduce S. hermonthica damage in sorghum in SSA, particularly in combination with other control options, such as intercropping, use of organic fertilizer and hand pulling of S. hermonthica at flowering to achieve integrated S. hermonthica management [35].
Genetic resistance: Striga resistance is the ability of the host root to stimulate Striga germination but at the same time prevent attachment of the seedlings to its roots or to kill the seedlings when attached. The use of resistant crop cultivars is the most economically feasible and environmentally friendly means of Striga control. In East Africa, the most promising new approach to Striga control is the use of resistant cultivars (e.g. of sorghum). Striga resistant cultivars have been bred in a number of crops. However, cultivars with immunity to Striga have not been found in all host crops. The host/parasite relationship is governed by a series of steps involving stimulation of germination, haustorium initiation, penetration of the host root, connection to the host xylem and concurrent growth [38].
Many cereals are found to be naturally resistant to Striga e.g.; rice, sorghum and some genotypes of maize. A resistant plant stimulates germination of Striga but it does not allow it to attach to the root. Study in Striga infested areas revealed cultivation with resistant crops results in fewerStriga plants and higher crop yield than a non-resistant genotype of the cultivated plant would do [39].
Biological control: Biological control is generally defined as the deliberate use of living organisms to suppress, reduce or eradicate a pest population [40]. Means of biological control of weeds include herbivorous insects, microorganisms specially fungi, and smothering plants. The insects that attack Striga can be classified according to the site damaged into defoliators such as Junonia spp., gall forming as Smicronyx spp., shoot borers as Apanteles spp., miners as Ophiomyia Strigalis, inflorescence feeders as Stenoptilodestaprobanes and fruit feeders as Eulocastra spp. [41]. Twenty eight fungi and two bacteria were found to be associated with Strigahermonthica in Sudan. Among the fungi, only Fusarium nygamai and Fusarium semitectum var. majus showed potential to be used as bio-agents for the control of Striga [42].
Chemical control: Various chemicals including herbicides, fumigants (e.g, methyl bromide) and germination stimulants (e.g, ethylene) have been reported as means of control of Striga [43]. Herbicides like Imazapyr and pyrithiobac applied as seed dressing to maize were reported to give efficient control of the parasite [44]. The excellent control capacity of the herbicides is most likely due to their relatively long persistence in the rhizosphere. Furthermore, multi-location testing showed that this herbicide provided excellent early season control of both S. asiatica and S. hermonthica and could increase yield 3 to 4-fold in heavy infested fields [44].
Emerged Striga plants can be successfully killed with common herbicides. However, much damage is done by the fully parasitic young plants before emergence, so such herbicide treatments do not necessarily reduce yield losses. The main strategy for control is accordingly to reduce the seed bank ofStriga in the soil by stimulating the seeds to germinate in the absence of host plants [45]. This can be achieved by:
- Planting a Poaceous trap crop (susceptible cereal or grass) which is ploughed in a few weeks after sowing before the weeds mature and set seed;
- Sowing crops which stimulate germination, but are not parasitized, for several seasons (e.g. sunflower, groundnut, soybean);
- Treating the soil with ethylene which simulates the chemical substances which exude from host roots and stimulate germination.
An integrated management approach, if properly designed, using a combination of suitable control measures, has the potential to provide a lasting solution to Striga problems. [10] reported that soil fertility and soil moisture management should be an integral part of any Striga control strategy. A similar study by [50] pointed out that species of Striga were controlled by using the resistant variety, fertilizer and tied ridges on farms of eastern Ethiopia which had long been abandoned due to Striga infestation. According to Table 2, species of Striga were controlled by using the resistant variety, fertilizer and tied ridges on farms; whereas, the local cultivar had severe infestations where the average yield of the resistant variety was 1718 kg ha-1 as against only 216 kg ha-1 from the local variety. The Striga-infested local varieties died, failed to produce a head or had very small heads. [51] also added that treatment combination that included resistant variety, fertilizer and tied ridge gave significantly higher yield followed by one that combined local variety with fertilizer and tied ridging in North wolloat Sirinka and Kobo sites.
Table 1: Effect of variety, intercropping and nitrogen rate interaction on the severity of Striga infestation (%)
| Intercropping/ Maize Variety | Nitrogen rate (kgNha-1) | |||
|---|---|---|---|---|
| 0 | 50 | 100 | Mean | |
| Maize only (J0-98) – Resistant | 62.2 | 39.2 | 9.4 | 36.9 |
| Maize only (Local) –Susceptible | 82.2 | 40.4 | 30.4 | 51.0 |
| J0-98 + Soyabean | 19.4 | 14.2 | 6.8 | 13.5 |
| Local + Soyabean | 50.1 | 20.8 | 10.8 | 27.2 |
| J0-98 + groundnut | 13.2 | 6.2 | 4.3 | 7.9 |
| Local + Groundnut | 45.2 | 19.3 | 7.6 | 24.0 |
| N-rate mean | 45.4 | 23.4 | 11.6 | - |
Source: Intercrops With Trap Crops, Nitrogen Fertilization for Striga hermonthica (Del.) Benth Control at Niger State [49].
Table 2: Striga count and sorghum yield as influenced by variety, fertilizer and tied ridge
| Treatment | Striga count/m2 | Yield (kg/ha) | ||||
|---|---|---|---|---|---|---|
| Babile | Fedis | Gursum | Babile | Fedis | Gursum | |
| Improved variety with fertilizer and tied ridge | 1 | 2 | 4 | 1467 | 1740 | 1947 |
| Improved variety without fertilizer and tied ridge | 2 | 3 | 5 | 1200 | 980 | 1244 |
| Local variety with fertilizer and tied ridge | 140 | 151 | 170 | 122 | 235 | 290 |
| Local variety without fertilizer and tied ridge | 266 | 181 | 288 | 98 | 148 | 130 |
Striga count against treatment and yield against treatment were significant at p = 0.01. Striga count against location and yield against location were not significant
Source: Distribution of two Striga species and their relative impact on local and resistant sorghum cultivars in East Ethiopia [50].
REFERENCES
- FAO (Food and Agricultural Organization). Investing in Sustainable Agricultural Intensification. The Role of Conservation Agriculture.A Framework for Action. Technical report: 2008.
- Alemie A, KeesstraSD and Stroosnijder L. A new agro-climatic classification for crop suitability zoning in northern semi-arid Ethiopia.Agricultural and Forest Meteorology, 2010; 150: 1057–1064.
- CASL. Arid and Semi-arid lands: characteristics and importance. Community Adaptation and Sustainable Livelihoods: 2006.
- Rezene F and Etagegnehu GM. Development of Technology for the Dryland Farming Areas of Ethiopia (Reddy, N.S. and Giorgis, Kidane Eds.). Proc. of 1st Nat. Workshop on Dryland Farming Res. in Ethiopia, 1994; 26-28 Nov. 1991.
- Parker C. Observations on the current status of Orobacheand Striga problems worldwide. Pest ManagSci, 2008; 65: 453- 459.
- Gethi JG and Smith ME. Genetic responses of single crosses of maize to Striga hermonthica (Del.) Benth and Striga asiatica (L.) Kuntze. Crop Sci, 2004; 44: 2068- 2077.
- Matusova R, Rani K, Verstappen FWA, Franssen MCR, Beale MH and Bouwmeester HJ. The strigolactone germination stimulants of the plant- parasitic Striga and Orobanche spp. are derived from the carotenoid pathway. Plant Physiology, 2005; 139: 920- 934.
- Aly R. Conventional and biotechnological approaches for control of parasitic weeds. Invitro Cell. Dev. Biol- Plant, 2007; 43:304- 317.
- AATF (African Agricultural Technology Foundation). Feasibility Study on Striga Control in Sorghum. African Agricultural Technology Foundation, Nairobi: 2011: ISBN 9966- 775-12-9.
- Babiker AGT. Striga: The Spreading Scourge in Africa. Regul. Pl. Grow. and Devel, 2007; 42: 74-87.
- Gomez-Roldan V, Fermas S, Brewer PB,Puech-Pagès V, Dun EA, Pillot JP, Letisse F, Matusova R, Danoun S, Portais JC, Bouwmeester H, Bécard G, Beveridge CA, Rameau C, and Rochange SF. "Strigolactone inhibition of shoot branching". Nature, 2008; 455:180-194.
- Cardoso C, Ruyter-Spira C, Bouwmeester HJ. Strigolactones and root infestation by plant-parasitic Striga, Orobanche and Phelipanche spp. Plant Sci, 2010; 180:414-420.
- Babiker AGT, Butler LG, Ejeta G and Woodson WR. Ethylene biosynthesis and strigol-induced germination of Strigaasiatica. Physiol. Plant, 1993; 88: 359-365.
- Berner DK, Winslow MD, Awad AE, Cardwell KF, Mohan Raj DR, and Kim SK.(eds.). Striga Research Methods: A manual 2nd edition.International Institute of Tropical Agriculture, PMB 5320, Ibadan, Nigeria: 1997.
- Ejeta G, LG Butler and AGT Babiker.New Approaches to the Control of Striga.Striga Research at Purdue University. Research Bulletin, 1993; 991:27.
- Oswald A. Striga control technologies and their dissemination. Crop Protection, 2005; 24: 333-342.
- Press MC and Scholes JD. Current status and future prospects for management of parasitic weeds (Striga and Orobanche). In: CR Riches (ed.). The World’s Worst Weeds. Brighton, British Crop ProtectionCouncil. Proc. of an Int. Symp: 2001: pp. 71- 90.
- Esilaba AO. Options for Striga management in Kenya .Kenya Agricultural Research Institute, Nairobi, Kenya: 2006.
- De Groote H, Wangare L, Kanampiu F, Odendo M and Friesen D. Potential markets for herbicide resistant maize seed for Striga in Africa. Back ground paper for a poster presented at the European Asso. Of Agric Economists congress, Copenhagen, Denmark: 23-27 August, 2005.
- Bilalis D, Papastylianou P, Konstantas A, Patsiali S, Karkanis A and Efthimiadou A. Weed-suppressive effects of maize-legume intercropping in organic farming. Int J Pest Manag, 2010; 56:173-181.
- Khan ZR, Pickett JA, Hassanali A, Hooper AM and Midgea CAO. Desmodium species and associated biochemical traits for controlling Striga species: Present and future prospects. Weed Research, 2008; 43: 302- 306.
- Odhiambo GD, and Ransom JK. Effect of dicamba on the control of Striga hermonthica in maize in western Kenya. African Crop Science Journal, 1993; 1:105-110.
- Midega CAO, Khan ZR, Amudavi DM, Pittchar J and Pickett JA. Integrated management of Striga hermonthicaand cereal stem borers in finger millet [Eleusinecoracana (L.) Gaertn.] through intercropping with Desmodiumintortum. International Journal of Pest Management, 2010; 56:145-151.
- Fasil R, Verkleij JAC, and Ernst WHO. Intercropping for the Improvement of Sorghum Yield, Soil Fertility and Striga Control in the Subsistence Agriculture Region of Tigray (Northern Ethiopia).Journal of Agronomy and Crop Science, 2005; 191:10—19
- Carsky RJ, Berner DK, Oyewole BD, Dashiell K and Schulz S. Reduction of Striga hermonthica parasitism on maize using soybean rotation. International Journal of Pest Management, 2000; 46:115–120.
- Parker C and Riches CR. Parasitic Weeds of the World: Biology and Control. Wallingford CAB International: 1993, p:332.
- Pieterse AH, JA Verkleij. Effect of Soil Condition on Striga Development –a Review .In: JK Ransom, LJ Musselman, AD Worsham and C Parker (Eds.), Striga. Proceedings of the Fifth International Symposium of Parasitic Weeds, April 1991. CIMMYT, Nairobi, Kenya: 1991, pp: 329-339.
- Vogt W, Saurborn J and Honisch M. Strigahermonthica, distribution and infestation in Ghana and Togo on grain crops. In: Ransom JK, Musselman LJ, Worsham AD and Parker C (Eds.), Proceedings of the Fifth International Symposium on Parasitic Weeds. CIMMYT, Nairobi, Kenya: 1991: pp. 272-277.
- Hess DEand Ejeta G. Effect of cultural treatment on infestation of Striga hermonthica(L.) Benth (Scrophulariaceace). In: Weber HC and Forstreuter W (eds.). Proceedings of the fourth international; symposium on parasitic flowering plants. Phillips University, Marburg Germany: 1987, p: 367375.
- Mumera LM and Below FE .Role of nitrogen in resistance to Striga parasitism of maize.In: Esilaba AO, F Reda, Ransom JK, WondimuBayu, GebremehdinWoldewahid and BeyeneshZemichael. Integrated Nutrient Management Strategies For Soil Fertility Improvement and Striga Control in Northern Ethiopia.African Crop Science Journal, 1993; 8 (4): 403- 410.
- Kamara AY, Ekeleme F, Menkir A, Chikoye D and Omoigui LO. Influence of nitrogen fertilization on the performance of early and late maturing maize cultivars under natural infestation with Striga hermonthica. Archives of Agronomy and Soil Science,2009; 55(2):125–145.
- Dzomeku IK and Murdoch AJ.Effects of prolonged conditioning on dormancy and germination of Striga hermonthica.Journal of Agronomy, 2007; 6:29-36.
- Jamil M, Charnikhova T, and Cardoso C. Quantification of the relationship between strigolactones and Striga hermonthica infection in rice under varying levels of nitrogen and phosphorus. Weed Research, 2011; 51:373–385.
- Lopez-Raez JA, Charnikhova T, Gomez-Roldan V. Tomato strigolactones are derived from carotenoids and their biosynthesis is promoted by phosphate starvation. New Phytologist, 2008; 178:863–874.
- Jamil M, Kanampiu FK, Karaya H, Charnikhova T and Bouwmeester HJ.Striga hermonthica parasitism in maize in response to N and P fertilizers.Field Crops Research, 2012; 134, 1–10.
- Simier P, Constant S, and Degrande D. Impact of nitrate supply in C and N assimilation in the parasitic plant Striga hermonthica (Del.) Benth (Scrophulariaceae) and its host Sorghum bicolor L. Plant Cell and Environment, 2006; 29:673–681.
- Tabo R, Bationo A, Gerard B. Improving cereal productivity and farmers’ income using a strategic application of fertilizers in West Africa. In: A Bationo, B Waswa, JKiharaand JKimetu (eds). Advances in Integrated Soil Fertility Management in Sub-Saharan Africa: Challenges and Opportunities. Springer, Dordrecht, the Netherlands: 2007, 201– 208.
- Butler LG. Chemical Communication between the Parasitic Weed Striga and its host crop, a new dimension in allelo-chemistry, In: Ml Dakshini and FA Einhelling (eds). Allelopathy: Organisms, Processes, and Application. ACS Symposium Series 582, American Chemical Society. Washington DC., USA: 1993, .158-168.
- Rodenburg J, Bastiaans L, and Kropff MJ. Characterization of host tolerance to Striga hermonthica.Euphytica. In: Larsson M. Soil fertility status and Striga hermonthica infestation relationship due to management practices in Western Kenya. (MSc Thesis Online publication: http://stud.epsilon.slu.se), SLU, Swedish University of Agricultural Sciences, 2006; (http://stud.epsilon.slu.se/4488/1/larssonm120704.pdf).Acces sed on April 21/2014
- BoyetchkoSM. Innovative application of microbial agents for biological weed control. In: KG Mukerji, BP Chamola and RKUpdahyay (eds.). Biotechnological Approaches in Bio control of Plant Pathogens. Kluwer Academic Plenum, New York, USA: 1999: pp. 73-97.
- Kroschel J. Analysis of the Striga problem, towards joint action. In: J Kroschel, H Mercer-Quarshie, and J Sauerborn (eds). Advances in Parasitic Weed Control at On-farm Level, 1:13–26. Joint Action to Control Striga in Africa. Marggraf- Verlag, Weikersheim, Germany: 1999.
- Zahran EB. Biological Control ofStriga hermonthica (Del.) Benth. Using Formulated Mycoherbicides Under Sudan Field Conditions. Ph.D Thesis, University of Hohenheim, Germany: 2008, pp:143.
- Egley GH, Eplee RE and Norris RS. Discovery and testing of ethylene as a witchweed germination stimulant. In: PF Sand, RE Eplee, and RG Westbrooks (eds). Witch weeds Research and Control in the United States. Weed Science Society of America Campaign, 1990; 37-45.
- Kanampiu FK, Kabamble V, Massawe C, Jasia L, Friesen D, Ransom JK and Gressel J. Multi-site, multi-season field tests demonstrate that herbicide seed-coating, herbicide-resistance maize controls Striga spp. and increases yield in several African countries. Crop Prot, 2003; 22: 697 - 706.
- Hesammi E. Striga and Ways of Control.Intl.J.Farm and Alli.Sci, 2013; 2(3):53-55. (http://ijfas.com/wpcontent/ uploads/2013/02/53-55.pdf). Accessed on April 21, 2014.
- Marley PS, Aba DA, Sheboyan JAY, Musa R and Sanni A. Integrated management in Striga hermonthica in sorghum using a mycoherbicide and host plant resistance in the Nigerian Sudano-Sahelian Savanna. Weed Res, 2004; 44: 157 – 162.
- Franke AC, Ellis-Jones J,Tarawali G, Schulz S, Hussaini MA, Kureh I, White R, Chikoye D, Douthwaite B, Oyewole BD and Olanrewaju AS. Evaluating and scaling-up integrated Strigahermonthica control technologies among farmers in northern Nigeria. Crop Protection,2006; 25:868– 878.
- Kamara AY, Chikoye D, Ekeleme F, Omoiguil O and Dugje IY.Field performance of improved cowpea cultivars under natural infestation with Striga gesneroides.International Journal of Pest Management,2008; 54(3):189–195.
- Mamudu AY. Intercrops With Trap Crops, Nitrogen Fertilization For Striga hermonthica (Del.) Benth Control At Niger State. International Journal of Humanities and Social Science Invention, 2013; 2(5):64-69.
- Temam T. Distribution of two Striga species and their relative impact on local and resistant sorghum cultivars in East Ethiopia. Wiley Inter Science, Trop. Sci, 2006; 46(3), 147– 150. DOI: 10.1002/ts.70. Accessed on April 21, 2014.
- Gebisa E and Gressel J (Eds).Integrating New Technologies For Strigacontrol: Towards Ending the Witch-Hunt.World Scientific Publishing Co. Pte. Ltd., Singapore: 2007, p:345.
| International Journal of Life-Sciences Scientific Research (IJLSSR)
Open Access Policy
Authors/Contributors are responsible for originality, contents, correct
references, and ethical issues.
IJLSSR publishes all articles under Creative Commons
Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |