ABSTRACT- This study was an attempt to estimate the prevalence of fungal isolates in superficial mycoses cases attending IPD and
OPD of IIMS&R, Lucknow, Uttar Pradesh.A prospective study over a period of six (6) months was conducted from January 2015 to
June 2015.The suspected cases of superficial mycoses were subjected to mycological examination with direct microscopy using 10%-
40% KOH depending on the types of samples (skin, nail, hair) processed and culture on Sabouraud’s dextrose agar with chloramphenicol
and cycloheximide (SDCCA) and also on Potato dextrose agar (PDA) medium. Causative agents were identified macroscopically
and microscopically from the growth obtained on SDCCA and PDA. Direct microscopy revealed fungal elements in 78 (66.1%)
cases whereas 54 (45.7%) were positive on culture. Out of 54 (45.7%) culture positive samples 6 (15%) were negative on microscopy
(KOH mount). Tineacorporis 38 (32.2%) was the most common clinical types and male is to female ratio in relation to clinical types
was 2.2:1. Commonest age group affected were 21-30 years with 41 (34.7%) cases. Males were predominantly affected 41 (75.9%)
and male to female ratio being 3.1:1. 60% of the patients came from the rural background. College students formed a major chunk of
the cases 29 (24.6%) followed by housewives 18 (15.3%) and unskilled workers 16 (13.6%). Trichophyton mentagrophytes 20 (37%)
was the predominat isolate followed by T. tonsurans15 (27.7%), T. rubrum3 (5.5%), M. audouinii 3 (5.5%) and T. schoenleinii 2
(3.7%) with no Epidermophyton species. A non-pigmented variant of T. rubrum was identified in this study. Both SDCCA and PDA
were found equally effective in isolating fungal isolates from clinical samples in our study. We are reporting change in frequency of
dermatophytes isolated from superficial mycoses cases in our region.
Key words- Superficial mycoses, Non-pigmented variants, Dermatophytes
INTRODUCTION
Superficial mycoses refer to the disease of the skin and its appendages
caused by the fungi. This group includes dermatophytosis,
pityriasis versicolor, tinea nigra, white piedra, black piedra and
candidiasis1. These fungi have the capability to produce keratinase,
which allow them to metabolize and live on human keratin
like skin,hair and nail2. Dermatophyte infections are one of the
earliest known infection of mankind and are very common
throughout the world3.Although dermatophytosis do not produce
mortality, it does cause morbidity and poses a major public health
problem, especially in tropical countries like India due to the hot
and humid climate3.Infection of skin or nail can also be of non
dermatophytic fungi and yeast-like fungi. Over the last decades,
an increasing number of non dermatophytes filamentous fungi
have been recognized as agents of skin and nail infections in humans,
producing lesions clinically similar to those caused by
dermatophytes4. The causative fungi colonize only the cornified
layer of the epidermis or supra-follicular portions of hair and do
not penetrate into deeper anatomical sites.
MATERIALS AND METHODOLOGY
A prospective study over a period of six (6) months from January
2015 to June 2015 was conducted at Integral Institute of Medical
Sciences & Research, Lucknow, Uttar Pradesh. The study population
comprised of 118 clinically suspected cases of superficial
mycoses attending Dermatology outpatients department at
Integral Institute of Medical Sciences And Research, Lucknow.
All the clinically suspected cases of superficial mycoses referred
to Department of Microbiology for isolation and identification of
etiological agent were included in the study.Demographic details
of every case and detailed history of onset of disease, duration of
symptoms, trauma, occupation, drugs, associated co-morbid con-
ditions, family and personal history was taken. Enquiries were
also made as to exposure to animals, cases or any other suspected
sources.
SPECIMEN COLLECTION AND PROCEDURE-
The affected areas were swabbed with 70% alcohol. Skin scrapings
or nail clippings or plucked hair was collected in clean white
paper packets.
Skin scraping:
Skin scrapings were collected by scraping across the inflammatory
margin of the lesion including the healthy skin using sterile
scalpel or clean slide. If vesicles are present, the top was removed
with fine scissors and stored for further examination.
Nail scraping:
Nail specimen was collected by taking the infected nail clippings
and was scraped deeply enough to obtain the recently invaded
nail tissue. In cases of paronychia i.e. where a yeast infection is
suspected, exudate was expressed from the paronychial folds by
probing with a flat excavator and collecting on a swab previously
moistened with sterile saline.
Hair plucking:
Hair specimen was collected by plucking the infected hair including
the base of hair shaft. The species most frequently associated
with scalp ringworm cause the affected hairs to flouresce under a
wood’s lamp and this is a useful means of selecting material.
Direct microscopic examination
KOH mount:
10% KOH solution was used for skin and hair
samples. 40% KOH for nail specimens and incubated overnight
at 370C for clearing.Clearing can be hastened by gently heating.
As soon as the specimen has cleared, examined under microscope
using the 10x and 40x objectives, for the presence of filamentous,
septate, branching hyphae with or without arthrospores. In case
of hair, type and arrangement of spores were noted (ectothrix/
endothrix). Tinea versicolor infections were diagnosed by the
presence of round yeast cells with short, stout and curved hyphae
(spaghetti and meat ball appearance).
Gram stain: Gram staining was done when growth of yeast
like fungi is suspected.
Fungal culture-
All the samples were collected and inoculated on two sets of test
tubes containing Sabouraud’s dextrose agar with chloramphenicol
and cycloheximide and Potato dextrose agar. For Tineaversicolor
infections SDA with sterile olive oil overlay was used. The fungal
cultures have been identified by colony morphology, rate of
growth and pigment production. Lactophenol cotton blue mount
was done from the small bit of colony taken on clean glass slide
and teased out using two teasing needles, to detect the presence
of macroconidia, microconidia, chlamydospore and special hyphal
structures. Confirmatory identification of the species was
done by slide culture technique and Biochemical tests i.e. urease
test, hair perforation test and rice grain test. Speciation of yeast
like fungi was done by gram’s stain, germ tube test, sugar fermentation
and assimilation tests10-14.
OBSERVATION AND RESULTS
A total of 118 patients were enrolled in the study, comprising 82
males (69%) and 36 females (31%). None of them had any systemic
diseases. Tinea corporis 38 (32.2%) was the most common
clinical type seen (table 1) and the male to female ratio in relation
to clinical types was found to be 2.2:1 which was significant
(p=0.00) (table 2). And the predominant age group affected was
21-30 years 41 (34.7%) and say that males were affected more
than female. 81(68.6%) of patients were literate, 74 (62.7%) cases
belonged to low socio-economic status and 60% of cases were
from rural area. College students formed a major chunk of the
cases 29 (24.6%) followed by housewives 18 (15.3%) and unskilled
workers 16 (13.6%).
Table 1: Clinical types in total samples of superficial
mycoses
| S. No. |
Clinical Types | Total n (%) |
| 1. |
Tinea corporis | 38 (32.2) |
| 2. |
Tinea pedis | 17 (14.4) |
| 3. |
Tinea manuum | 17 (14.4) |
| 4. |
Tinea cruris | 15 (12.7) |
| 5. |
Tinea unguium | 8 (6.8) |
| 6. |
Tinea faceii | 8 (6.8) |
| 7. |
Tinea capitis | 5 (4.2) |
| 8. |
Pityriasis versicolor | 5 (4.2) |
| 9. |
Tinea barbae | 4 (3.4) |
| 10. |
Bulbous tineapedis | 1 (0.8) |
| 11. |
Total | 118 (100.0) |
Table 2: Sex distribution of clinical types of superficial mycoses
Cases
| CLINICAL TYPES | TOTAL n (%) | MALE n (%) | FEMALE n (%) | M:F RATIO | P -VALUE |
| Tinea corporis | 38 (32.2) | 26 (31.7) | 12 (33.3) | 2.1:1 | < .05 |
| Tinea pedis | 17 (14.4) | 9 (11.0) | 8 (22.2) | 1.1:1 | 0.808* |
| Tinea manuum | 17 (14.4) | 11 (13.4) | 6 (16.7) | 1.8:1 |
0.225* |
| Tinea cruris | 15 (12.7) | 13 (15.9) | 2 (5.6) | 6.5:1 |
N.A. |
| Tinea unguium | 8 (6.7) | 6 (7.3) | 2 (5.6) | 3:1 |
N.A. |
| Tinea faceii | 8 (6.7) | 5 (6.1) | 3 (8.3) | 1.6:1 |
N.A. |
| Tinea capitis | 5 (4.2) | 4 (4.9) | 1 (2.8) | 4:1 |
N.A. |
| Pityriasis versicolor | 5 (4.2) | 4 (4.9) | 1 (2.8) | 4:1 |
N.A. |
| Tinea barbae | 4 (3.3) | 4 (4.9) | 0 (0.0) | 4:0 |
N.A. |
| Bulbous tineapedis | 1 (0.8) | 0 (0.0) | 1 (2.8) | NA |
N.A. |
| Total n (%) | 118 (100) | 82 (69.5) | 36 (30.5) | 2.2:1 |
< .05 |
N.A- not applicable due to low sample size, (p=0.05=significant), (*=insignificant)
Out of total 118 clinical samples processed, found 78 (66.1%) were positive by direct microscopy (KOH mount) and 54 (45.7%) were
culture positive. Whereas, 30 (38.4%) samples were found to be KOH positive but culture negative, while 6 (15%) were KOH negative
samples were culture positive. 34 (85%) samples were negative by both KOH and culture (table 3). Culture negative samples
were excluded while calculating the prevalence of fungal isolates.
Table 3: Comparative results of KOH & culture
| KOH RESULTS n (%) |
CULTURE |
| POSITIVE n (%) |
NEGATIVE
n (%) |
| POSITIVE | 78 (66.1) | 48 (61.5) | 30 (38.4) |
| NEGATIVE | 40 (33.9) | 6 (15.0) | 34 (85.0) |
| TOTAL | 118 (100) | 54 (45.7) | 64 (54.2) |
Out of total 54 culture positive cases 41 (76%) were male patients and 13 (24%) were female patients, male to female ratio was 3.1:1
which was significant (p=0.00) (Table 4).
Among 54 (45.7%) culture positive isolates T. mentagrophytes 20 (37%) was the commonest isolate followed by T.tonsurans15
(27.7%), T.rubrum 3 (5.5%), M. audouinii 3 (5.5%), T. schoenleinii 2 (3.7%), Aspergillus terreus 2 (3.7%), Alternaria species 2
(3.7%), Fusarium species 2 (3.7%), Candida albicans 2 (3.7%), Candida non-albicans 1 (1.8%), Scytalidium species 1 (1.85%) and
Mucor species 1 (1.8%) (Figure1).
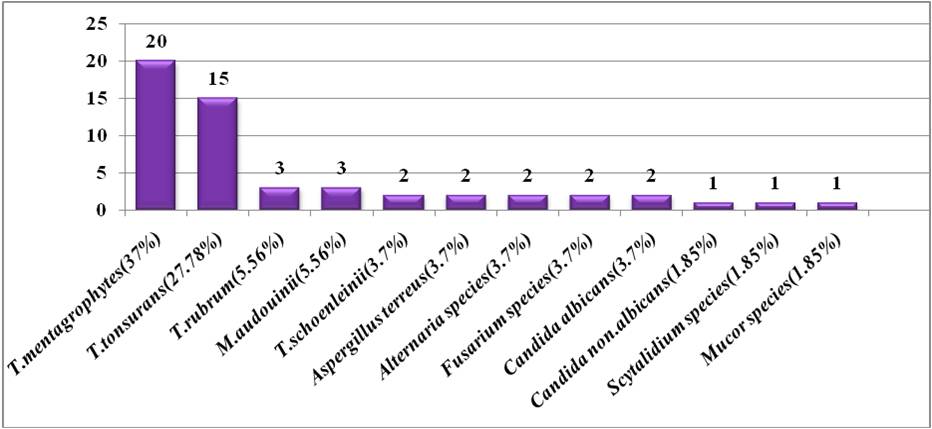
Figure 1: Bar diagram showing frequency of fungal isolates from culture positive samples
Table 4: Distribution of fungal isolates in relation to sex
| FUNGAL ISOLATES | n (%) | MALE n (%) | FEMALE n (%) | P VALUE |
| T. mentagrophytes | 20(37) | 16 (39) | 4 (30.8) | < .05 |
| T. tonsurans | 15 (27.8) | 13 (31.7) | 2 (15.4) | N.A |
| T. rubrum | 3 (5.6) | 3 (7.3) | 0 (0.0) | N.A |
| T. schoenleinii | 2 (3.7) | 1 (2.4) | 1 (7.7) | N.A |
| M. audouinii | 3 (5.6) | 1 (2.4) | 2 (15.4) | N.A |
| Aspergillus terreus | 2 (3.7) | 2 (4.9) | 0 (0.0) | N.A |
| Alternaria species | 2 (3.7) | 1 (2.4) | 1 (7.7) | N.A |
| Fusarium species | 2 (3.7) | 2 (4.9) | 0 (0.0) | N.A |
| Candida albicans | 2 (3.7) | 1 (2.4) | 1 (7.7) | N.A |
| Candida non-albicans | 1 (1.9) | 0 (0.0 | 1 (7.7) | N.A |
| Scytalidium species | 1 (1.9) | 0 (0.0) | 1 (7.7) | N.A |
| Mucor species | 1 (1.9) | 1(2.4) | 0 (0.0) | N.A |
| TOTAL n (%) | 54 (100) | 41 (100) | 13 (100) | < .05 |
N.A-non applicable due to low sample size, p=0.05=significant
DISCUSSION-
Out of total 118 cases of superficial mycoses, 78 (66.1%) were positive in direct microscopic examination (KOH). 54 (45.7%) were
culture positive. Similar findings were reported in other studies also, which is mentioned in (Table 5).
Table 5: Comparison of KOH positivity between present and other studies
| Present
study |
Madhulika et al.,15
2014
(West Bengal) |
Vyas et al.,16 2013
(North India) |
Singh and Beena.,17
2003
(Baroda) |
Bindu et al.,18
2002
(Calicut) |
| KOH
POSITIVE |
66.1% | 75.7% | 62.5% | 60.3% | 64% |
| KOH NEGATIVE | 33.9% | 24.3% | 37.5% | 39.7% | 36% |
We found an isolation rate of 48 (61.5%) among the samples which showed positive KOH mount. Out of 54 (45.7%) culture positive
samples 6 (15%) were negative on microscopy (KOH mount). Similar findings were reported in other studies which are mentioned in
(Table 6).
Table 6: Comparison of culture positivity between present and other studies
| Present
study |
Madhulikaet al.,15
2014
(West Bengal) |
Bhatia and Sharma.,19
2014
(Shimla) |
Vyas et al.,16
2013
(North India) |
Singh and Beena.,
17
2003
(Baroda) |
| CULTURE
POSITIVE |
45.7% | 46.28% | 36.6% | 37.5% | 44.62% |
In the present study,Trichophyton mentagrophytes was the predominate isolate followed by T. tonsurans, T. rubrum, M. audouiniiand
T. schoenleiniiwith the frequency of 37%, 27.7%, 5.5%, 5.5% and 3.7% respectively. However, we did not observe any involvement
of Epidermophyton species in the study.
Majority of the studies have reported T. rubrum as main dermatophytic isolate from superficial mycoses cases in India17; 20-24,. However,
we have found T. mentagrophytes as predominant species followed by T. tonsurans and T. rubrum. Our findings are similar to recent
studies done by others 19, 25, 26, and hints towards change in frequency of dermatophytes in our region.
Table 7: Showing fungal isolates of present and other studies performed in India
| Isolate | Present
study |
Agarwal et al.,25
2014
(North-west India) |
Bhatia & Sharma.,19
2014
(Himachal Pradesh) |
Sahai & Mishra.,26
2011
(Central India) |
| T. mentagrophytes | 37% | 37.9% | 63.5% | 25% |
| T. tonsurans | 27.7% | 8.3% | - | 20% |
| T. rubrum | 5.5% | 34.2% | 35.1% | 5% |
| T. schoenleinii | 3.7% | - | - | 7% |
| M. audouinii | 5.5% | - | - | 5% |
CONCLUSION
Our study has given us insights into the clinic-mycological aspects
of superficial mycoses in our region. The study reveals that
skin infections are more common than the hair and nail infections
in dermatophytoses cases. Common clinical types are T. corporis,
T. pedis, T. manuum and T. cruris. Unhygienic conditions among
low-socioeconomic group, frequent migration of labourers,
workers to this region may be some of the contributing epidemiological
factors.Dermatomycoses was seen in 94.5% and superficial
candidiasis in 5.5% cases. T. mentagrophytes was implicated
asthe predominating species followed by T. tonsurans, T. rubrum,
M. audouinii, T. schoenleinii, Aspergillus terreus, Alternaria species,
Fusarium species, Candida albicans, Candida non-albican,
Scytalidium species and Mucor species. Isolation ofnon pigmented
variants of T. rubrum confirms our findings of change in
distribution of dermatophytes in our region. India is a growing
economy and during last couple of decades interstate migration
of population and increase in national and foreign tourism might
be an important reason for change in frequency of dermatophytes
and uncommon fungal isolates in clinical practice. We are reporting
a change in frequency of dermatophytosis isolated from superficial
mycoses cases in our region. However, the present study
is a small study that focuses primarily on the prevalence of different
dermatophytes species in Northern Lucknow and a systematic
study covering larger population and over a longer period
of time would give a better insights into the epidemiology of
dermatophytes in Lucknow and neighboring region.
REFERENCES
- Grover W C S and Roy C P (Clinico-mycological profile Of Superficial
Mycoses in a Hospital in North- East India).Medical Journal
Armed Forces India, 2003; 59:2:114-116.
- Das K, Basak S and Ray S (A Study on Superficial Infection from
West Bengal: A brief Report). J Life Sc 2009; 1:1:51-55.
- Venkatesan G,Ranjit Singh A J A, Murugesan A G, Janaki C and
Gokul ShankarS (Trichophytonrubrum-the predominant etiological
agent in human dermatophytoses in Chennai,India). AfrJMicrobiol
Res, 2007; 9-12.
- Aggarwal A, Arora U and Khanna S (Clinical and Mycological
Study of Superficial Mycoses in Amritsar).Indian J Dermatol,
2002; 47:4:218-220.
- Ananthnarayan R and Paniker, textbook of medical microbiology.
2013; 595-599.causes of white piedra of scalp hair. Indian J
DermatolVenereolLeprol, 2014; 80:324-7.
- Koneman E W, Color Atlas and Text book of Diagnostic Microbiology,
Lippincott Williams and Wilkins, 2006, 6, 1187.
- Zaias N (Superficial white onychomycosis).Sabouraudia 1966; 5:
99-103.
- Franger P and Belson I (ScopulariopsisBainier as causative agent
ofonychomycosis).ActaUniverCorolilaeMedic,. 1974: 20: 333-338.
- Li M, Chen Q, Shen Y and Liu W (Candida albicansphospholipomannan
triggersinflammatory responses of human keratinocytes
through Toll-like receptor 2). ExpDermatol. 2009 Jul. 18(7):603-10.
- Winn WJ, Allen S, Janda W, Koneman E, Procop G, Schreckenberger
P,Woods G. Koneman’scolour Atlas and textbook of diagnostic
Microbiology, 6th edition. Philadelphia: Lippincott Williams and
Wilkins, 2006; 1156-1171, 1187-1192.
- Rippon JW. Medical Mycology, 3rdedition. Philadelphia London:
W.B.Saunders Co., 1974:169-275.
- Chander J. Textbook of Medical Mycology, 3rd edition India: Mehta
publishers, 2009. p. 91-122, 389-396.
- Bailey and Scott. Diagnostic microbiology, 12th edition 2007;
629-695.
- Mackie & McCartney Practical medical microbiology, 14th ed.;
Churchill Livingstone; 2012:695-708.
- Madhulika A. Mistry et.al Int J Biol Med Res. 2014; 5(4): 4556-
4561.
- Vyas A, Pathan N, Sharma R and Vyas L (A clinic-mycological
study of cutaneousmycoses in Sawai Man Singh Hospital of Jaipur,
North India). Ann Med Health SciRes, 2013; 3:593.
- Singh S and Beena PM (Comparitive study of different microscopic
techniques and culture media for the isolation of dermatophytes).
Indian J Med Microbiol, 21:21–24
- Bindu V and Pavithran K (Clinico - mycological study of dermatophytosis
in Calicut). Indian J Dermatol Venereol Leprol, 2002;
68:259-61.
- Bhatia and Sharma (Epidemiological studies on Dermatophytosis in
humanpatients in Himachal Pradesh, India). SpringerPlus 2014
3:134.
- Kumar Y, Singh K, Kanodia S, Singh S and Yadav N (Clinicoepidemiological
profile of superficial fungal infections in
Rajasthan). International Medical Journal March 2015; 2(3):
139 -143.
- Pandey A and Pandey M (Isolation and characterization of dermatophytes
with tineas infection at Gwalior (M.P.), India). Int J Pharm
SciInvestig, 2013; 2(2):05–08.
- Balakumar S, Rajan S, Thirunalasundari T and Jeeva S
(Epidemiology of dermatophytosis in and around Tiruchirapalli,
Tamilnadu, India). Asian Pac J., 2012.
- Patel P, Mulla S, Patel D and Shrimali G (A study of superficial
mycosis in south Gujarat region). Nat J Commun Med Res., 2010,
(2):85–88.
- Mathur M, Baradkar V P, De A, Taklikar S and Gaikwad S (Dermatomycosis
caused by common and rare fungi in Mumbai). Indian J
DermatolVenereolLeprol, 2008; 74 (4): 402-403.
- Agarwal US, Saran J and Agarwal P (Clinico-mycological study of
dermatophytes in a tertiary care centre in northwestIndia). Indian J
DermatolVenereolLeprol, 2014; 80:194.
- Sahai S, Mishra D (Change in spectrum of dermatophytes isolated
from superficial mycoses cases: first report from central
India).Indian Journal ofDermatology, Venereology, and Leprology,
2011, 77(3): 335-36.
International Journal of Life-Sciences Scientific Research (IJLSSR)
Open Access Policy
Authors/Contributors are responsible for originality, contents, correct
references, and ethical issues.
IJLSSR publishes all articles under Creative Commons
Attribution- Non-Commercial 4.0 International License (CC BY-NC).
https://creativecommons.org/licenses/by-nc/4.0/legalcode |
