Int. J. Life. Sci. Scienti.
Res., 1(2): 44-51, November 2015
Daily Rhythms of Oxygen
Consumption in Freshwater Crab (Barytelphusa jaquemontii) & Prawn (Macrobranchium lamerrii)
Sudha Bansode*
Associate Professor, Department of Zoology, Shankarrao Mohite Mahavidyalaya, Akluj, Maharashtra, India
*Address for Correspondence: Sudha Bansode, Associate Professor, Department of Zoology, Shankarrao
Mohite College, Maharashtra, India
ABSTRACT- Biological
rhythms are the equal combination of ecological & physiological events
producing the internal sense of time in living being. The internal metabolic
rate is influenced by the degree of voluntary activity that is affected by
environmental conditions & is associated with changing season time of day
or month and body size Bliss and Montel (1968) found that, in general, the
smaller individuals within a species or a small sized the species have higher
metabolic rate per unit rate and time then larger animals. Dehnel
and wines (1960) observed the distinct diurnal rhythm of oxygen consumption the
rhythm is characterized that maximum utilization is at 8.00 to 9.00 a.m. by a
second smaller peak is at 10.00 to 11.00 p.m. at midnight. Several workers
carried out such type of studies [Diwan and Nagbhushnam (1972)]. The crab, B. jaquemontii were collected and kept
for laboratory acclimatization. The pH & temperature were 7.2
& 150C respectively. All the crabs were in the size range was
3.0 cm. to 7.0 cm. The experiments were performed of 30 animals and the oxygen
consumption of each individual was measured by Wrinkler’s
Method (1960).
Key Word: Oxygen consumption, aquatic respiration, respiration, oxygen
INTRODUCTION- Numerous water
breathers exhibit a gas exchange regulation strategy that maintains 0(2)
partial pressure, P (Oxyzen) in the arterial blood
within the range 1- 3 kpa at rest during the daytime.
In night active crustacean, they examined weather this could limit the rate of
0(2) consumption CM (02) of locomotor muscles and /
or the whole body as part of a coordinated response to energy conservation. Carvalho et al (1997) showed the routine metabolic and
ammonia excretion rates were measured during minimum but quantities in the
shrimp, Xiphopenaeus kroyeri
at five different temperatures (20, 22, 25, 28, 300C) in a flow through
system. The animals rapidly achieved uniform rates, showing little handling
stress, which may represent an experimental artifact that is responsible for
wide variation in the measurement of routine rates. A circadian rhythm of R and
U rate, was detect as they were significantly higher during dark conditions.
MATERIALS AND
METHODS- The crab (B. jacquemontii) & prawn (M. lamerrii)
were collected and kept for laboratory acclimatization. The pH and temperature
were 7.2 and 150C respectively. All the crabs were in the size range
was 3.0 cm to 7.0 cm. The experiments were performed 30 animals. The oxygen
consumption of individual animals was measured by Wrinkler’s
method (1960). The crabs were allowed to settle for 24 hrs before recordings
were made, and then transferred within 1 hr. to experimental jar. The oxygen
consumption measurement was made from morning 9.00 to 10.00 a.m. and
measurements were performed 2, 4, 6, 24 and 48 hrs under circadian clock.
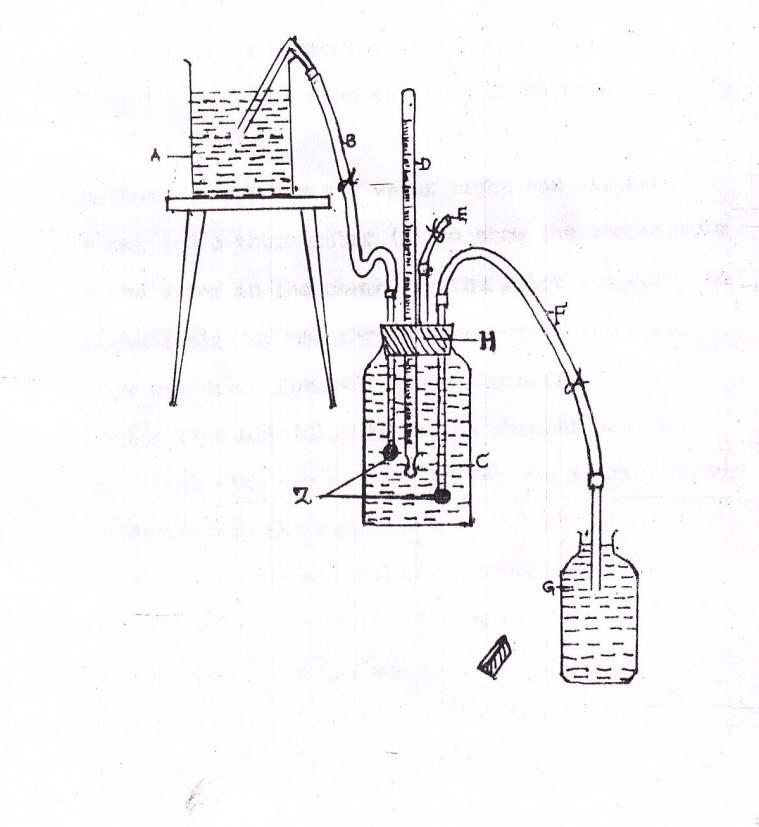 A -
Reservoir
A -
Reservoir
B -
Inlet tube
C -
Respiratory chamber
D -
Thermometer
E -
Air tight tube
F -
Out-let tube
G -
Sample collecting bottle
H -
Rubber cork
Fig. 1: Diagram showing the
arrangement of the respiratory chamber
Experiments
Effect of
temperature on oxygen consumption of Crab
(Bertelphusa jaquemontii )-
The crab were taken in each weight
group i.e. between 0.310 and 0.320 gms and smaller
prawns weighting between 0.180 to 0.190 gms, and were
exposed to the freshwater pre- adjusted to varying temperatures (thermostatically
adjusted). Oxygen consumption was measured at 150, 200,
250, and 300C. The room temperature during the course of
experiment was 25 + 10C. The results are represented in Table
1A. The Table shows that as the temperature decreased from the control (25 +
10C), the rate of oxygen consumption decrease steadily, but as the
temperature increased, the rate of oxygen consumption also increased. Table 1 B
indicates the values of Q10. In the bigger prawns Q 10 at high
temperature (25 + 300C) was 2.978 and at low temperature (15 +
250C) was 1.809, whereas in the smaller Crabs, Q10 at
high temperature (25 + 300C) was 1.482 and at low temperature
(15 + 250C) was 1.239.
Oxygen
consumption of Berytelphusa jaquemonttii at
different sodium chloride concentrations-
The prawns are exposed to five different concentrations of NaCl;
0.1%, 0.2%, 0.3%, 0.4% and 0.5%. The first sets of observations were made using
from the stock aquarium containing tap water. This served as control. After
measuring the oxygen consumption of prawns in normal tap water, experiments
were conducted on prawns exposed to above mentioned salt concentrations. The
results are given in Table 2. It is evident from the result that oxygen
consumption increases steadily from 0.1% to 0.5% of salt concentration.
Influence of pH
of the media on oxygen consumption- The
oxygen consumption were determined at 9 different pH media, i.e. 5.0, 5.5, 6.5
(Control), 7.0, 7.5, 8.0, 8.5, 9.0 and 9.5 respectively, at the laboratory
temperature. The results given in Table 3, demonstrate that in acidic pH the
oxygen consumption decreased when compared with the control, at pH 7.0 and 7.5,
the respiration did not show much variation. However, high alkaline pH tends to
decrease the oxygen uptake.
The influence of
oxygen tension of the medium on the oxygen consumption- The oxygen consumption of the prawn was measured at
six different oxygen concentrations i.e. 1.6; 2.5, 3.5, 4.6, 5.4, ml/1. The
results presented in Table 4 reveals that oxygen consumption did not vary much
between oxygen tension of 4.6 to 6.5 ml/1. At low oxygen tension of the medium,
the rate of oxygen consumption decreased considerably. With the rise in oxygen
tension the rate of oxygen consumption increased.
Effect of decision on oxygen
consumption- The oxygen consumption
of normal crabs was measured first and this soured as control. 10 prawns were
taken from the stock aquaria, blotted thoroughly with filter paper and exposed
to the atmospheric air. Every 15 minutes intervals the crabs exposed to the
atmospheric air were taken and their oxygen consumption was measured by patting
them respirometer. The results are shown in table 5.
It is seen from the table that the rate of oxygen consumption was increased as
the time of exposure to the atmospheric air increased.
Effect pf
starvation on oxygen consumption- The
results are shown in Table 6. It is seen that the oxygen consumption was
reduced to nearly 50% after starving the Crab for 14 days. The oxygen
consumption went on decreasing as the days of starvations increased.
Oxygen consumption in relation to
body weight- Metabolism varies
according to the body weight and sex. This experiment was conducted on Crab of
both to sexes separately and the results are shown in Table 7 A and 7 B, table
7 A, shows the results of weight specific oxygen consumption of female prawns
and that of table 7 B, shows the results of weight specific oxygen consumption
of male prawns. The female prawns weighing between 0.168 to 0.357 gms were grouped at 0.024 gms
intervals (average) and their respiratory rates were measured. Likewise, male
prawns belonging to the weight groups of 0.075 gms to
0.50 gms were chosen for the experiments. They were
grouped t 0.012 gms intervals (average) and their
respiratory rates were measured.
The
weight specific oxygen consumption for smaller female Crabs (0.168 gms) was found to be 0.51 + 0.004 ml/ gm/h/1,
whereas for the bigger female prawns (0.357 gms), it
was 0.015 + 0.007 ml/gm/1 whereas for the bigger individuals (0.150 gms), the rate of oxygen consumption was found to be 0.045 +
0.06 ml/gm/h/1. Thus, the experiments clearly demonstrated that the rate of
oxygen consumption was decreased as the body weight increased irrespective of
sex of the individuals.
Oxygen consumption in relation to
sex- In this experiment male
and female Crabs pf almost of same weight groups were
taken and their oxygen consumption was measured. The results are given in Table
8. It is quite obvious from the table that the rate of oxygen consumption was
found to be more in the male prawns than the females.
Diurnal rhythm in oxygen
consumption of Bertelphusa jaquemontii -
The results are given in Table 9.
It is evident from the table that the rate of oxygen
consumption was maximum at 8.00 a.m. and minimum at 8.00 p.m. Oxygen
consumption went on increasing from 8. P.m. and reached its peak at 8.00 a.m.
and then slowly declined by 8.00 p.m. so there appears to be a distinct rhythm
in oxygen consumption of Bertelphusa jaquemontii.
Effect
of photoperiod on oxygen consumption- The data is given in
the Table 10. It is seen the table that the oxygen consumption is maximum in
the prawns exposed to 24 hrs light. However, the prawns exposed to 18 hrs light
and 6 hrs darkness and Vice-Versa showed slight change in the oxygen
consumption over the control.
RESULTS
Effect of
temperature on oxygen consumption of Macrobrachium lamerrli- The
prawns were taken in each weight group i.e. between 0.310 and 0.320 gms and smaller prawns weighting between 0.180 to 0.190 gms, and were exposed to the freshwater pre-adjusted to
varying temperatures (thermostatically adjusted). Oxygen consumption was
measured at 150, 200, 250 and 300C.
The room temperature during the course of experiment was 25 + 10C.
The results are represented in Table 1A. The Table shows that as the temperature
decreased from the control (25 + 10C), the rate of oxygen
consumption decrease steadily, but as the temperature increased, the rate of
oxygen consumption also increased.
Table-
1 A Effect of temperature and weight on oxygen consumption of Berytelphusa jaequemontiii
Temperature : 25 + 10C
PH : 3.6
Oxygen tension : 6.9 ml/1
Sex : Female
|
S. No. |
Average weight of prawns (in gms) |
Temperature of the dedium in (0C) |
Oxygen consumption Ml/g/h/1 + S.D. |
|
1 |
0.315 |
0.315 |
0.034
+ 0.008 |
|
2 |
0.315 |
0.315 |
0.046-
+ 0.004 |
|
3 |
0.315 |
0.315 |
0.051
+ 0.009 |
|
4 |
0.315 |
0.315 |
0.088
+ 0.005 |
|
5 |
0.185 |
0.185 |
0.073
+ 0.004 |
|
6 |
0.185 |
0.185 |
0.181
+ 0.002 |
|
7 |
0.185 |
0.185 |
0.092
+ 0.007 |
|
8 |
0.185 |
0.185 |
0.011
+ 0.008 |
Table
1 B indicates the values of Q10. In the bigger prawns Q10
at high temperature (25 + 300C) was 2.978 and at low
temperature (15 + 250C) was 1.809, whereas in the smaller
prawns, Q10 at high temperature (25 + 30OC) was
1.482 and at low temperature (15 + 250C) was 1.239.
Table
-1 B Q10 Oxygen consumption of Macrobrachium lamerrii as a function of weight and
temperature
|
S.
No. |
Weight of the prawn (in grams) |
Q 10 at |
||
|
15-20Oc |
20-25Oc |
25-30Oc |
||
|
1 |
0.315 |
1.809 |
1.229 |
2.978 |
|
2 |
0.185 |
1.231 |
1.290 |
1.482 |
Table-
1 Effect of different sodium chloride
on oxygen consumption of Berytelphusa jaequemontiii
Temperature : 25 + 10C
PH : 3.6
Oxygen tension : 6.9 ml/1
Sex : Female
|
S. No. |
Average weight of Crabs (in gms) |
Temperature of the dedium in (oC) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
215.10 |
15 |
10.034
+ 0.034 |
|
2 |
210.20 |
20 |
10.046-
+ 0.001 |
|
3 |
210.25 |
25
(Control) |
09.051
+ 0.034 |
|
4 |
200.30 |
30 |
8.088
+ 08.088 |
|
5 |
175.25 |
15 |
7.073
+ 07.073 |
|
6 |
165.20 |
20 |
6.081
+ 06.081 |
|
7 |
160.15 |
25
(Control) |
5.092
+ 05.092 |
|
8 |
157.10 |
30 |
4.011
+ 04.011 |
Table-
1 B Q 10 Oxygen consumption of Berytelphusa jaequemontiii as a function of weight
and temperature
|
S. No. |
Weight of the
Crab (in grams) |
Q 10 at |
||
|
15-200C |
20-250C |
25-300C |
||
|
1 |
0.215 |
1.809 |
1.229 |
2.978 |
|
2 |
0.285 |
1.231 |
1.290 |
1.482 |
Table-
1 A Effect of temperature and weight on oxygen consumption of Berytelphusa jaequemontiii
Temperature : 25 + 10C
PH : 3.6
Oxygen tension : 6.9 ml/1
Sex : Female
|
S. No. |
Average weight of Crabs (in gms) |
Oxygen consumption + S.D. Ml/g/h/1 |
|
1 |
|
0.044
+ 0.006 |
|
2 |
0.1 |
0.046-
+ 0.004 |
|
3 |
0.2 |
0.054
+ 0.005 |
|
4 |
0.3 |
0.064
+ 0.007 |
|
5 |
0.4 |
0.095
+ 0.008 |
|
6 |
0.5 |
0.106
+ 0.005 |
Oxygen consumption of Macrobrachium lamerrii at
different sodium chloride concentrations- The
prawns are exposed to five different concentrations of NaCl;
0.1%, 0.2%, 0.3%, 0.4% and 0.5%. The first sets of observations were made using
from the stock aquarium containing tap water. This served as control. After
measuring the oxygen consumption of prawns in normal tap water, experiments
were conducted on prawns exposed to above mentioned salt concentrations. The
results are given in Table 2. It is evident from the result that oxygen
consumption increases steadily from 0.1 % to 0.5 % of salt concentration.
Table- 2 Effect of different sodium
chloride consumptions on the oxygen consumption of Macrobrachium lamerrii
Temperature : 26 + 1Oc
Oxygen tension : 4.6 ml/1
PH : 6.8
Average weight : 0.268 gms
Sex : Female
|
S. No. |
Salinity (X) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
Control |
0.044
+ 0.006 |
|
2 |
0.1 |
0.046
+ 0.004 |
|
3 |
0.2 |
0.054
+ 0.005 |
|
4 |
0.3 |
0.064
+ 0.007 |
|
5 |
0.4 |
0.095
+ 0.008 |
|
6 |
0.5 |
0.106
+ 0.005 |
Table- 2 Effect of different sodium
chloride consumptions on the oxygen consumption of Berytelphusa jaequemontiii
Temperature : 26 + 10C
Oxygen tension : 4.6
ml/1
PH : 6.8
Average weight : 0.168 gms
Sex : Female
|
S. No. |
Salinity (X) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
Control |
10.044
+ 0.006 |
|
2 |
0.1 |
10.046
+ 0.004 |
|
3 |
0.2 |
10.054
+ 0.005 |
|
4 |
0.3 |
10.064
+ 0.007 |
|
5 |
0.4 |
10.095
+ 0.008 |
|
6 |
0.5 |
10.106
+ 0.005 |
Influence of pH of the media on
oxygen consumption- The oxygen
consumption was determined at 9 different pH media, i.e. 5.0, 5.5, 6.5 (Control), 7.0, 7.5, 8.0, 8.5,
9.0 and 9.5 respectively at the laboratory temperature. The results given in
Table 3, demonstrate that in acidic pH the oxygen consumption decreased when
compared with the control, at pH 7.0 and 7.5, the respiration did not show much
variation. However, high alkaline pH tends to decrease the oxygen uptake.
Table- 3: Effect of the different pH of the media on
oxygen consumption of Macrobrachium lamerrii
Temperature : 27
+ 10C
PH of H2O : 6.5
Oxygen tension : 6.69 ml/1
Weight : 0.265 gms
Sex : Female
|
S. No. |
Salinity (x) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
6.4
Control |
0.024
+ 0.007 |
|
2 |
5.0 |
0.017
+ 0.006 |
|
3 |
5.5 |
0.019
+ 0.005 |
|
4 |
7.0 |
0.024
+ 0.006 |
|
5 |
7.5 |
0.023
+ 0.008 |
|
6 |
8.0 |
0.021
+ 0.004 |
|
7 |
8.5 |
0.018
+ 0.006 |
|
8 |
9.0 |
0.015
+ 0.007 |
|
9 |
9.5 |
0.012
+ 0.008 |
Table-
3: Effect of the different pH of the
media on oxygen consumption of Berytelphusa jaequemontiii
Temperature : 27 + 10C
PH of H2O : 6.5
Oxygen tension : 6.69 ml/1
Weight : 265 gms
Sex : Female
|
S. No. |
Salinity (x) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
6.4
Control |
10.024
+ 0.007 |
|
2 |
5.0 |
10.017
+ 0.006 |
|
3 |
5.5 |
10.019
+ 0.005 |
|
4 |
7.0 |
10.024
+ 0.006 |
|
5 |
7.5 |
10.023
+ 0.008 |
|
6 |
8.0 |
10.021
+ 0.004 |
|
7 |
8.5 |
10.018
+ 0.006 |
|
8 |
9.0 |
10.015
+ 0.007 |
|
9 |
9.5 |
10.012
+ 0.008 |
The influence of oxygen tension of
the medium on the oxygen consumption- The
oxygen consumption of the prawn was measured at six different oxygen concentrations
i.e. 1.6, 2.5, 3.5, 4.6, 5.4, ml/1. The results presented in Table 4 reveals
that oxygen consumption did not vary much between oxygen tension of 4.6 to 6.5
ml/1. At low oxygen tension of the medium, the rate of oxygen consumption
decreased considerably. With the rise in oxygen tension the rate of oxygen
consumption increased.
Table- 4: Effect of the oxygen tension of the medium on
the oxygen consumption of Macrobrachium lamerrii
Temperature
: 26 + 10C
pH
of H2O : 6.8
Oxygen
tension : 4.6 ml/1
Average Weight : 0.270 gms
Sex : Female
|
S. No. |
Salinity (X) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
1.6 |
0.006
+ 0.007 |
|
2 |
2.5 |
0.022
+ 0.006 |
|
3 |
3.5 |
0.042
+ 0.008 |
|
4 |
4.6
Control |
0.069
+ 0.005 |
|
5 |
5.4 |
0.071
+ 0.005 |
|
6 |
6.5 |
0.073
+ 0.007 |
|
7 |
7.0 |
0.077
+ 0.006 |
Table- 4: Effect of the oxygen
tension of the medium on the oxygen consumption of Berytelphusa jaequemontiii
Temperature : 26 + 10C
pH of H2O : 6.8
Oxygen tension : 4.6 ml/1
Average Weight : 270 gms
Sex : Female
|
S.
No. |
Salinity
(X) |
Oxygen
consumption + S.D. Ml/gm/h/1 |
|
1 |
1.6 |
11.006
+ 0.007 |
|
2 |
2.5 |
10.022
+ 0.006 |
|
3 |
3.5 |
11.042
+ 0.008 |
|
4 |
4.6
Control |
09.069
+ 0.005 |
|
5 |
5.4 |
10.071
+ 0.005 |
|
6 |
6.5 |
08.073
+ 0.007 |
|
7 |
7.0 |
07.077
+ 0.006 |
Effect of decision on oxygen
consumption- The oxygen consumption
of normal crabs was measured first and this soured as control. 10 prawns were
taken from the stock aquaria, blotted thoroughly with filter paper and exposed
to the atmospheric air. Every 15 minutes intervals the crabs exposed to the
atmospheric air were taken and their oxygen consumption was measured by patting
them respirometer. The results are sown in table 5.
It is seen from the table that the rate of oxygen consumption was increased as
the time of exposure to the atmospheric air increased.
Table
- 5: Effect of desiccation on the oxygen
of Macrobrachium lamerrii
Temperature : 26 + 10C
Average Weight : 0.268 gms
Sex : Female
|
S.
No. |
Salinity
(X) |
Oxygen
consumption + S.D. Ml/gm/h/1 |
|
1 |
Control |
0.056
+ 0.005 |
|
2 |
15 |
0.061
+ 0.007 |
|
3 |
30 |
0.070
+ 0.008 |
|
4 |
45 |
0.079
+ 0.006 |
|
5 |
60 |
0.088
+ 0.007 |
|
6 |
75 |
0.096
+ 0.005 |
|
7 |
90 |
0.108
+ 0.008 |
|
8 |
105 |
0.177
+ 0.006 |
|
9 |
120 |
0.123
+ 0.006 |
Table
- 5: Effect of desiccation on the oxygen
of Berytelphusa jaequemontiii
Temperature : 26 +
10C
Average Weight : 268 gms
Sex : Female
|
S. No. |
Salinity (X) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
Control |
10.056
+ 0.005 |
|
2 |
15 |
10.061
+ 0.007 |
|
3 |
30 |
10.070
+ 0.008 |
|
4 |
45 |
10.079
+ 0.006 |
|
5 |
60 |
10.088
+ 0.007 |
|
6 |
75 |
09.096
+ 0.005 |
|
7 |
90 |
08.108
+ 0.008 |
|
8 |
105 |
07.177
+ 0.006 |
|
9 |
120 |
06.123
+ 0.006 |
Effect of starvation on oxygen
consumption- The results are shown
in Table 6. It is seen that the oxygen consumption was reduced to nearly 50%
after starving the Crab for 14 days. The oxygen consumption went on decreasing
as the days of starvations increased.
Table
- 6: Effect of the starvation on the
oxygen consumption of Macrobrachium lamerrii
Temperature : 26 + 10C
pH of H2O : 6.7
Oxygen tension : 5.04 ml/1
Average Weight : 0.267 gms
Sex : Female
|
S. No. |
Days of starvation |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
Control |
0.056
+ 0.005 |
|
2 |
2 |
0.051
+ 0.004 |
|
3 |
4 |
0.045
+ 0.006 |
|
4 |
6 |
0.040
+ 0.007 |
|
5 |
8 |
0.037
+ 0.008 |
|
6 |
10 |
0.033
+ 0.005 |
|
7 |
12 |
0.030
+ 0.007 |
|
8 |
14 |
0.028
+ 0.006 |
|
9 |
16 |
0.016
+ 0.008 |
|
10 |
18 |
0.010
+ 0.004 |
Table
- 6: Effect of the starvation on the
oxygen consumption of Berytelphusa jaequemontiii
Temperature : 26
+ 10C
pH of H2O : 6.7
Oxygen tension : 5.04 ml/1
Average Weight : 267 gms
Sex : Female
|
S. No. |
Days of starvation |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
Control |
10.056
+ 0.005 |
|
2 |
2 |
10.051
+ 0.004 |
|
3 |
4 |
10.045
+ 0.006 |
|
4 |
6 |
10.040
+ 0.007 |
|
5 |
8 |
10.037
+ 0.008 |
|
6 |
10 |
10.033
+ 0.005 |
|
7 |
12 |
09.030
+ 0.007 |
|
8 |
14 |
07.028
+ 0.006 |
|
9 |
16 |
05.016
+ 0.008 |
|
10 |
18 |
04.010
+ 0.004 |
Oxygen consumption in relation to
body weight- Metabolism varies according
to the body weight and sex. This experiment was conducted on Crab of both to
sexes separately and the results are shown in Table 7 A and 7 B, table 7 A,
shows the results of weight specific oxygen consumption of female prawns and
that of table 7 B, shows the results of weight specific oxygen consumption of
male prawns. The female prawns weighing between 0.168 to 0.357 gms were grouped at 0.024 gms
intervals (average) and their respiratory rated were measured. Likewise, male
prawns belonging to the weight groups of 0.075 gms to
0.50 gms were chosen for the experiments. They were
grouped t 0.012 gms intervals (average) and their
respiratory rates were measured.
The
weight specific oxygen consumption for smaller female Crabs (0.168 gms) was found to be 0.51 + 0.004 ml/ gm/h/1,
whereas for the bigger female prawns (0.357 gms), it
was 0.015 + 0.007 ml/ gm/h/1. In the caser of
male prawns, the weight specific oxygen consumption for smaller individuals
(0.075 gms) was found to be 0.092 + 0.007 ml/
gm/1 whereas for the bigger individuals (0.150 gms),
the rate of oxygen consumption was found to be 0.045 + 0.06 ml/gm/h/1. Thus,
the experiments clearly demonstrated that the rate of oxygen consumption was
decreased as the body weight increased irrespective of sex of the individuals.
Table- 7 A: Effect of the different body weights and sex
on the oxygen consumption of Macrobrachium lamerrii
Temperature : 26 + 10C
pH of H2O : 6.8
Oxygen tension : 5.02 ml/1
Average Weight : 0.267 gms
Sex : Female
|
S. No. |
Average body weight of a prawn (In grams) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
0.168 |
0.051
+ 0.004 |
|
2 |
0.180 |
0.047
+ 0.006 |
|
3 |
0.201 |
0.045
+ 0.007 |
|
4 |
0.228 |
0.042
+ 0.006 |
|
5 |
0.248 |
0.036
+ 0.005 |
|
6 |
0.267 |
0.033
+ 0.008 |
|
7 |
0.282 |
0.026
+ 0.008 |
|
8 |
0.321 |
0.020
+ 0.006 |
|
9 |
0.357 |
0.015
+ 0.007 |
Table-
7 B: Effect of the different body weights and sex on the oxygen consumption –
Prawn.
Temperature : 27 + 10C
pH of H2O : 6.7
Oxygen tension : 5.26 ml/1
Average Weight : 0.267 gms
Sex : Male
|
S. No. |
Average body weight (In grams) |
Oxygen consumption +
S.D. Ml/gm/h/1 |
|
1 |
0.075 |
0.092
+ 0.007 |
|
2 |
0.084 |
0.088
+ 0.008 |
|
3 |
0.098 |
0.073
+ 0.005 |
|
4 |
0.011 |
0.065
+ 0.008 |
|
5 |
0.125 |
0.058
+ 0.007 |
|
6 |
0.137 |
0.050
+ 0.005 |
|
7 |
0.150 |
0.045
+ 0.006 |
Table - 7 A: Effect of the different body weights and sex
on the oxygen consumption of Berytelphusa jaequemontiii
Temperature : 26 + 10C
pH of H2O : 6.8
Oxygen tension : 5.02 ml/1
Average Weight : 267 gms
Sex : Female
|
S. No. |
Average body weight of a Crab (In grams) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
168 |
10.051
+ 0.004 |
|
2 |
180 |
10.047
+ 0.006 |
|
3 |
201 |
10.045
+ 0.007 |
|
4 |
228 |
10.042
+ 0.006 |
|
5 |
248 |
10.036
+ 0.005 |
|
6 |
267 |
10.033
+ 0.008 |
|
7 |
282 |
09.026
+ 0.008 |
|
8 |
321 |
06.020
+ 0.006 |
|
9 |
357 |
04.015
+ 0.007 |
Table- 7 B: Effect of the different
body weights and sex on the oxygen consumption Berytelphusa jaequemontiii
Temperature : 27 + 10C
pH of H2O : 6.7
Oxygen tension : 5.26 ml/1
Average Weight : 267 gms
Sex : Male
|
S. No. |
Average body weight (In grams) |
Oxygen consumption +
S.D. Ml/gm/h/1 |
|
1 |
75 |
10.092
+ 0.007 |
|
2 |
84 |
10.088
+ 0.008 |
|
3 |
98 |
10.073
+ 0.005 |
|
4 |
11 |
10.065
+ 0.008 |
|
5 |
25 |
06.058
+ 0.007 |
|
6 |
137 |
05.050
+ 0.005 |
|
7 |
150 |
04.045
+ 0.006 |
Oxygen consumption in relation to
sex- In this experiment male
and female Crabs of almost of same weight groups were taken and their oxygen
consumption was measured. The results are given in Table 8. It is quite obvious
from the table that the rate of oxygen consumption was found to be more in the
male prawns than the females.
Table
- 8: Effect of sex on the oxygen consumption of jaequemontii
Temperature : 27 + 10C
pH of H2O : 6.7
Oxygen tension : 5.26 ml/1
Average Weight : 0.267 gms
Sex : Male
|
S. No. |
Sex |
Average body weight (In grams) |
Oxygen consumption + S.D. Ml/gm/h/1 |
|
1 |
Male |
0.166 |
0.062 +
0.005 |
|
|
Female |
0.168 |
0.051 +
0.008 |
|
2 |
Male |
0.198 |
0.052 +
0.006 |
|
|
Female |
0.200 |
0.074 +
0.007 |
|
3 |
Male |
0.226 |
0.049 +
0.007 |
|
|
Female |
0.228 |
0.072 +
0.005 |
|
4 |
Male |
0.247 |
0.040 +
0.006 |
|
|
Female |
0.248 |
0.036 +
0.008 |
|
5 |
Male |
0.262 |
0.037 +
0.005 |
|
|
Female |
0.264 |
0.033 +
0.008 |
Table - 8:
Effect of sex on the oxygen consumption of Berytelphusa jaequemontiii
Temperature : 27 + 10C
pH of H2O : 6.7
Oxygen tension : 5.26 ml/1
Average Weight : 267 gms
Sex : Male
|
S. No. |
Sex |
Average body weight (In grams) |
Oxygen consumption +
S.D. Ml/gm/h/1 |
|
1 |
Male |
166 |
10.062
+ 0.005 |
|
|
Female |
168 |
10.051
+ 0.008 |
|
2 |
Male |
198 |
10.052
+ 0.006 |
|
|
Female |
200 |
09.074
+ 0.007 |
|
3 |
Male |
226 |
08.049
+ 0.007 |
|
|
Female |
228 |
08.072
+ 0.005 |
|
4 |
Male |
247 |
07.040
+ 0.006 |
|
|
Female |
248 |
07.036
+ 0.008 |
|
5 |
Male |
262 |
06.037
+ 0.005 |
|
|
Female |
264 |
06.033
+ 0.008 |
Diurnal rhythm in oxygen
consumption of Bertelphusa jaquemontii - The results are given in Table 9. It is
evident from the table that the rate of oxygen consumption was maximum at 8.00
a.m. and minimum at 8.00 p.m. Oxygen consumption went on increasing from 8.
p.m. and reached its peak at 8.00 a.m. and then slowly declined by 8.00 p.m. so
there appears to be a distinct rhythm in oxygen consumption of Bertelphusa jaquemontii.
Table - 9: Diurnal rhythm in oxygen consumption of Macrobrachium
lamerrii
Oxygen tension : 4.76 to 5.6 ml/1
pH of H2O : 6.9
Average Weight : 0.268 gms
Sex : Male
|
S. No. |
Average body weight (In grams) |
Oxygen consumption +
S.D. Ml/gm/h/1 |
|
1 |
8
a.m. |
0.028
+ 0.004 |
|
2 |
12
noon |
0.022
+ 0.006 |
|
3 |
4
p.m. |
0.021
+ 0.005 |
|
4 |
8
p.m. |
0.011
+ 0.006 |
|
5 |
12
mid night |
0.015
+ 0.007 |
|
6 |
4
a.m. |
0.028
+ 0.007 |
Table-
9: Diurnal rhythm in oxygen consumption of Berytelphusa jaequemontiii
Oxygen tension : 4.76 to 5.6 ml/1
pH of H2O : 6.9
Average Weight : 268 gms
Sex : Male
|
S. No. |
Average body weight (In grams) |
Oxygen consumption +
S.D. Ml/gm/h/1 |
|
1 |
8
a.m. |
10.028
+ 0.004 |
|
2 |
12
noon |
12.022
+ 0.006 |
|
3 |
4
p.m. |
10.021
+ 0.005 |
|
4 |
8
p.m. |
10.011
+ 0.006 |
|
5 |
12
mid night |
09.015
+ 0.007 |
|
6 |
4
a.m. |
07.028
+ 0.007 |
Effect of photoperiod on oxygen
consumption- The data is given in
the Table 10. It is seen from the table that the oxygen consumption is maximum
in the prawns exposed to 24 hrs light. However, the prawns exposed to 18 hrs
light and 6 hrs darkness and Vice-Versa showed slight change in the oxygen
consumption over the control.
Table-10: Effect of photoperiod on the oxygen
consumption of
Temperature : 27 + 10C
pH of H2O : 6.9
Oxygen tension : 4.92 ml/1
Average Weight : 0.270 gms
Sex : female
|
S. No. |
Average body weight (In grams) |
Oxygen consumption +
S.D. Ml/gm/h/1 |
|
1 |
12
D : 12 L (Control) |
0.053
+ 0.004 |
|
2 |
24
D : O L |
0.055
+ 0.005 |
|
3 |
12
D : 12 L |
0.057
+ 0.008 |
|
4 |
6
D : 18 L |
0.052
+ 0.007 |
|
5 |
18
D : 6 L |
0.054
+ 0.006 |
Table-10: Effect of photoperiod on the oxygen
consumption of Berytelphusa jaequemontiii
Temperature : 27 + 10C
pH of H2O : 6.9
Oxygen tension : 4.92 ml/1
Average Weight : 270 gms
Sex : female
|
S. No. |
Average body weight (In grams) |
Oxygen consumption +
S.D. Ml/gm/h/1 |
|
1 |
12
D : 12 L (Control) |
10.053
+ 0.004 |
|
2 |
24
D : O L |
10.055
+ 0.005 |
|
3 |
12
D : 12 L |
10.057
+ 0.008 |
|
4 |
6
D : 18 L |
10.052
+ 0.007 |
|
5 |
18
D : 6 L |
10.054
+ 0.006 |
REFERENCES
1) Carvalho P.P. (1997): Oxygen consumption and ammonia excrets of xiphopenaeus kroyari Heller shring oxygen
uptake and ammonia excretson of xiphopenaeus
kroyari Heller shrinrp
oxygen uptake and ammonia excreto.
2) Brown FA. Bennett M. P. and Webb H. M. (1954) : Daily and tidal rhythms of oxygen consumption
of fiddler crab. J. cell. Comp. Physiol 41477-506.
3) Bunning E.
(2003): The physidogical clock : circadian rhythmicity and biochronometry.
4) Brown
F.A. (1970): The biological clock: IIrd
edition Two views Academic press New York and London.
5) Brady J.
(1974): The physiology of insect
circadian rhythem Adv, Insect physiology 10; 1-115.
6) Cooke I. M.
(1988): Studies on the
crustaceans cadre gangues coup Blacker physic.
910 205 – 218.
7) DeCoursey P. J. (1976): “Biological rhythms in the marine envornment 283 pr. University of south carding press columma south carolins.”
8) DeCoursey P. J. (2000):
A circadian pacemaker in free- living chipmunks essentials for survivals
comp. Physiol. A 186 - 180.
9) DeCoursey P. S. (2001):
In zeitgebers. Entrainment and masking of the circadian
system, K. Honma and Honma (eds) pp. 55-74.
10) DeCoursey P. S. (2003):
The behavioral ecology and evolutron of mological timing systems. PP. 67-106 in chronobiology;
11) DeCoursey P. J. (1998):
Circadian performance and natural habitat a pilot study J. Bio. Rhythms 13 229
-244.
12) Diwan A. D. and Nagabhushanam
R. (1972): Influence of envornmental factors on oxygen consumption in the tropical
freshwater crab, Barytelphusa cunicularis C
(west wood) mearath. Univ. J. Sci. II (4); 131-146.
13) Diwan A. D. (1971):
Studies on the biology of the freshwater crab, Barytelphusa cunicularis C (west wood) Ph. D. Thesis,
Marathwada University, M.S. India.
14) Enright IT and Hammer W. M. (1967): Vertical diurnal migration and endogenous rhythmicity science 157.
937-941.
15) Enright IT (1970):
Ecological aspects of endogenons rhythmicity Annu. Rev. Eco. Syst. 1, 221-238.
16) Enright IT (1975):
Orientation in time: Endogenous
clocks Mar. Eco. 2 pp. 917 – 944.
17) Edney E. B. (1977):
Water balance in land arthropods J. springer verig Heideiberg 282 p.
18) Forgue J. Legeay A, Massabuan JC. (2001):
Is the resting rate of oxygen consumption in crustaceans limited by the
low blood oxygenation, strategy. J.
Expt. Bio. Vol. 204 pp. 933-940.
19) Hervant F (2004):
Adaptation to low oxygen in the Encyclopedia (editeur
: D: cucuer a. C. R. crumly)
Academic press New York PP 10-17.
20) Hervant F. (2005):
Metabolic responses in cold in subterranean crustaceans J. Expt. Bio. In
press.
21) Ramamurhti R. (1968):
Oxygen consumption of the common Indian cattle leech poecilobdella
grannulosa comp. Biochem
Physiol. 24, 283-287.
22) Veron P. (2002)
: Comparative study of the metabolic
responses during food shortage and subsequent recovery at different temperature
in the adull lesser mealworm physiol
Entomol. 27: 291 – 301.
23) Zwartz L. (1984)
: Wading animals in cranea
– Brssan winter 198283 Bull water study group P. 36 –
40.
24) Zenthen E. (1953) :
Oxygen uptake as related to body size in organisms quart Rev. Biol 28- 1 1-12