ABSTRACT- Background: Endometriosis is associated with chronic, benign, oestrogen-dependent inflammatory disease that affects approximately 10% of reproductive age women and 35-50% of women with pelvic pain and infertility. It can be a weakening disease with dysmenorrhoea, dyspareunia, and chronic pelvic pain symptoms.
Objective: To evaluate the role of serum marker (IL-6, IL-8, TNF-a) as non-invasive tool to diagnose endometriosis in reproductive age group.
Methods: A case control study was conducted in Department of Obstetrics and Gynecology, KGMU, Lucknow for a period of one year. Total numbers of women enrolled in study were 100. Out of 100 women, 75 women of reproductive age group with clinical suspicion and USG findings were taken as cases. Out of 75 cases, 12 cases lost the follow up and 26 cases kept on conservative management and they responded well. Finally 37 cases of endometriosis with strong clinical suspicions (Dysmenorrhea, Heavy or irregular bleeding, Pelvic pain, Lower abdominal or back pain, Dyspareunia, Dyschezia) and USG finding of endometriosis were recruited as cases and they underwent laparoscopy/ laparotomy. Control group comprises of 25 women undergoing for laparoscopic tubal ligation. After taking informed consent, all the women were subjected to the detailed menstrual, gynaecological, medical history and general, systemic and gynaecological examination. Patient was investigated for haemoglobin, ultrasound abdomen and pelvis and serum markers (IL-6, IL-8, TNF-a). Blood sample (5ml of blood) was collected in vecutainer tube for serum analysis. The blood was centrifuged to separate the serum and stored at -70ºC till examined. Finally 37 cases of endometriosis underwent laparotomy/laparoscopy (gold standard to diagnose endometriosis) for proper diagnosis and treatment.
Results: Serum IL-8 cut-off at 0.78% pg/ml afforded a sensitivity of 70.3% and specificity of 80% in the diagnosis of endometriosis and has good discriminant ability. TNF-a has average discriminant ability, 62.2% sensitivity and 56% specificity for endometriosis diagnosis. So that serum IL-8 and TNF-a can differentiate cases with or without endometriosis. By detecting these serum markers, we can diagnose endometriosis without undergoing laparoscopy or laparotomy.
Conclusion: The serum markers (IL-8, TNF-a) can be used as a non-invasive tool for diagnosis of endometriosis.
Key-words- Endometriosis, Interleukins, Tumour necrosis factor, Laparoscopy
INTRODUCTION
Endometriosis is the presence of endometrial like tissue (glands/stroma) outside the uterus which induces chronic inflammatory reaction. Endometriosis, multifactorial disease or syndrome that starts around pre pubertal age and flourishes after menarche with symptoms progressive in intensity. Susceptibility of endometriosis depends on complex interaction of immunological, hormonal, environmental & genetic factor.Endometriosis is typically seen during the reproductive years; it has been estimated that endometriosis occurs in roughly 6–10% of women.[1] The incidence of endometriosis in primary infertility is 20-30%, dysmenorrhea is 40-60% & of chronic pelvic pain is 71-80%. [2] Most common sites are pelvic viscera and peritoneum, out of which ovary is most commonly affected.
Symptoms may depend on the site of active endometriosis. Its main but not universal symptom is pelvic pain. Endometriosis is a common finding in women with infertility.[1]
The disease pattern of endometriosis is progressive dysmenorrhea (70%), pelvic pain (40%), infertility (35%), dyspareunia (33%), and menstrual irregularities (16%). Usually, the initial imaging examination for suspected endometriosis is pelvic ultrasound (US) scanning. Ultrasound is particularly helpful in the evaluation of endometriotic cysts but has a limited role in the diagnosis of adhesions or superficial peritoneal implants.[3] Magnetic resonance imaging (MRI) provides superior anatomic detail and better defines abnormalities found using ultrasonography.
Endometrial cells may self-generate via stem cells in specific niches of the endometrium[4]. Due to lack of hormone expression receptor undifferentiated endometrial stem cells may be less responsive to ovarian steroids than the terminally differentiated progeny. [5]
Peritoneal fluid of women with endometriosis contains soluble factors with angiogenic activity. [6] There is expanding proof that immunological mechanisms play a role in the pathogenesis and pathophysiology of endometriosis. It has been generally proposed that the peritoneal fluid microenvironment may contribute to endometriosis and/or endometriosis-associated infertility. Cytokines, immune-modulatory glycoproteins may mediate these processes.[7] It is suggested that activated T-cells produces cytokine products that may be involved in regulating cellular processes of endometriosis tissue. Elevated concentrations of interleukins IL and tumour necrosis factor alpha (TNF-a) have been observed in the peritoneal fluid of subfertile women with endometriosis as compared to fertile women without endometriosis suggesting cytokines may be involved in the progression of disease and infertility. [8-10]
Inflammation is key factor of endometriosis. Elevated TNF-a, in the peritoneal fluid of women with endometriosis, suggesting that TNF-a may involve in the aetiology and pathogenicity of this disease. [11] TNF regulates cellular proliferation and apoptosis via ligation of distinct TNF receptors (TNFR1 for apoptosis and TNFR2 for proliferation). [12]
Cytokines are diverse protein that plays a central role in regulating cell proliferation, activation, motility, adhesion, chemotaxis, and morphogenesis. They have been implicated in pathogenesis of endometriosis. [7] So, the this study was planned to diagnose the endometriosis by serum markers IL-6, IL-8, TNFa (non-invasive tool) and assess whether any of these markers can discriminate between patients with/without endometriosis.
MATERIALS AND METHODS
A prospective case control study was conducted in department of obstetrics and gynaecology, KGMU. The study included 100 patients admitted in the department of obstetrics and gynaecology, KGMU, Lucknow, India. Out of 100 women, 75 women of reproductive age group with clinical suspicion and USG findings were taken as cases and 25 women of reproductive age group, who were undergoing laparoscopic bilateral tubal ligation were taken as control. Out of 75 cases, 12 cases lost the follow up and 26 cases kept on conservative management and they responded well. Finally 37 cases of endometriosis underwent laparotomy/laparoscopy (gold standard to diagnose endometriosis) for proper diagnosis and treatment.
Inclusion criteria includes all women aged between 10-45 years and patients with symptoms of Menstrual abnormality, severe dysmenorrheal, Premenstrual pain, Chronic pelvic pain, Dyspareunia, GI complaints and Infertility was included in the study. Exclusion criteria were as follows patients with Acute Fever, Pelvic Inflammatory Disease (PID), Urinary Tract Infection (UTI), Chronic systemic illness (Diabetes Mellitus, Tuberculosis, Jaundice, Immuno-compromised).
Diagnosis and staging of endometriosis: Endometriosis was diagnosed during the laparoscopic procedure. The disease was staged according to the revised American Fertility Society (rAFS) classification. Laparoscopy was performed in post menstrual phase of cycle.
Collection of samples: A Blood sample (5 ml of venous blood) were collected preoperatively from all participants and was collected in vacutainer tube after taking all precautions to avoid haemolysis. The blood was centrifuged at 3000 rpm for 10 min at 4ºC to separate the serum and stored at -70?C until needed for analysis.
Serum IL-6, IL-8 and TNF-a cytokine concentrations were determined by using commercially available enzyme-linked immuno-sorbent assay (ELISA) Human, kit supplied by thermo fischer scientific. The assays employ the quantitative sandwich enzyme immunoassay technique using recombinant human IL-6, IL-8 and TNF-a, with antibodies raised against the recombinant proteins, respectively.
Analysis of Interleukin-6: According to manuals protocol (Thermo scientific kit: EH2IL6) assay employs an antibody specific for human IL-6 coated on a 96 well plate. Standard, samples, and biotinylated anti-human IL-6 are pipetted into the wells and IL-6 present in samples is captured by antibody immobilised to the well and by the biotinylated IL-6 specific detection antibody. After washing away unbound biotinylated antibody, HRP- conjugated streptavidin is pipetted to the wells. The well is again washed. Following this second wash step, TMB substrate solution is added to the wells, resulting in colour development proportional to the amount of IL-6 bound. The stop solution changes the colour from blue to yellow and the intensity of the colour is measured at 450 nm.
Analysis of Interleukin-8: According to manuals protocol (Thermo scientific kit: KHC0081) assay employs binding of IL-8 samples and known standards to the capture antibodies and subsequent binding of the biotinylated anti-IL-8 secondary antibody to the analyte is completed during the same incubation period. Any excess unbound analyte and secondary antibody is removed. The HRP conjugate solution is then added to every well including the zero well, following incubation excess conjugate is removed by careful washing. A chromogen substrate is added to the wells resulting in the progressive development of a blue coloured complex with the conjugate. The colour development is then stopped by the addition of acid turning the resultant final product yellow.
Analysis of Tumor necrosis factor a: According to manuals protocol (Thermo scientific kit: EH3TNFA) assay employs an antibody specific for human TNF-a coated onto a 96 well plate. Standard, samples and biotinylated anti human TNF-a is pipetted into wells. TNF-a present in a sample is captured by antibody immobilised to wells and by the biotinylated TNF-a specific detection antibody. After washing away unbound biotinylated antibody, HRP-conjugated streptavidin is pipetted to the wells. The well is washed again. Following second wash step, TMB substrate solution is added to the well, resulting in color development proportional to the amount of TNF-a bound. The stop solution changes the colour from blue to yellow and the intensity of the colour is measured at 450 nm.
STATISTICAL ANALYSIS
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 statistical Analysis Software. The values were represented in Number (%) and Mean±SD. Adjustment of p-value for multiple comparisons was done. Receiver operating characteristic (ROC) analysis was used to estimate the power of a cytokine to distinguish subjects with endometriosis from control and to choose an optimal cut off point for screening purpose.
RESULTS
A total number of 37 women underwent laparoscopy for infertility aged between 25-30 years. Out of the 37 patients with endometriosis, 29 (78.37%) had early disease (stages I and II) and 8 (21.62%) had late disease (stages III and IV). Subjects included in the study as Controls were having significantly higher (p<0.001) age(30.88+2.28 years) as compared to Cases (24.65+6.46 years) (Table-1).
Serum cytokines in subjects with endometriosis versus controls: We evaluated the concentration of three cytokines (Table-2) in the serum of subjects with endometriosis and controls Interleukin-6 (IL-6), Intreleukin-8(IL-8), Tumor necrosis factor-a (TNF-a). Serum IL-8 levels were found to significantly higher (p<0.001) in cases (0.95+0.52) as compared with controls (0.47+0.28). Serum cytokine IL-6 and TNF-a were of higher order in Cases than in controls but this difference was statistically non-significant (p>0.05).
Serum cytokines of subjects with endometriosis stratified by stage of the disease: Cytokines levels were marginally higher in early stage endometriosis than the advanced stage endometriosis. However, the difference between early and advanced stage endometriosis did not reach statistically significance for any of the measured cytokines.
Serum cytokines as potential markers for the non surgical prediction of endometriosis: In the multivariate regression analysis of all measured cytokines, serum IL-8 provided the best discriminative ability between subjects with endometriosis and controls (P>0.001).
For IL-8 area under curve = 0.803 (good discriminent ability) cut off >0.78 pg/ml provided a sensitivity of 70.3%, specificity of 80%, a positive likelihood ratio of 3.51% and negative likelihood ratio of 0.37%.
Table 1: Demographic characteristics of cases and controls
| Variable | Category | Controls | Cases | Total | OR (95% CI) | ‘p’ value |
|---|---|---|---|---|---|---|
| Age (Years) | Mean +SD (n) | 30.88 +2.28 (n=25) | 24.65 +6.46 (n=37) | 27.16 +6.01 (n=62) | (3.535-8.928) | <0.001 |
| Education | Illiterate | 13 | 6 | 19 | OR=2.80 (1.00-7.834) | 0.003 |
| Literate | 12 | 31 | 43 | |||
| Married | Married | 25 | 28 | 53 | 0.008 | |
| Unmarried | 0 | 9 | 9 | |||
| Amenorrhoea | Yes | 0 | 5 | 5 | 0.055 | |
| No | 25 | 32 | 57 | |||
| Dysmenorrhoea | Absent | 19 | 3 | 22 | OR=23.22 (5.101-105.728) | <0.001 |
| Present | 6 | 22 | 28 | |||
| Dyspareunia | Absent | 25 | 8 | 33 | <0.001 | |
| Present | 0 | 18 | 18 | |||
| Infertility | Absent | 25 | 16 | 41 | <0.001 | |
| Present | 0 | 21 | 21 |
Table 2: Infertility in study population
| Infertility | Cases (n=37) | Controls (n=25) | Statistical analysis | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | x2 | ‘p’ value | |
| Primary | 17 | 45.94 | 0 | 0.00 | 46.000 (df=2) | <0.001 |
| Secondary | 4 | 10.81 | 0 | 0.00 | ||
Table 3: USG finding in cases Population
| USG Reports | Cases (n=37) | |
|---|---|---|
| Number | % | |
| B/L ovarian endometriosis | 8 | 21.64 |
| Endometriosis of episiotomy scar | 1 | 2.70 |
| Endometriosis of POD | 7 | 18.92 |
| Endometriosis of POD & Lf ovary | 1 | 2.70 |
| Endometriosis of right adenxa | 1 | 2.70 |
| Endometriosis of right ovary | 2 | 5.41 |
| Endometriosis of right ovary and POD | 1 | 2.70 |
| Left ovarian cyst | 1 | 2.70 |
| Left ovarian chocolate cyst | 6 | 16.22 |
| Right ovarian endometriosis | 7 | 18.92 |
| Right To mass | 1 | 2.70 |
| Scar endometriosis | 1 | 2.70 |
Table 4: Serum cytokines (IL6, IL8, TNFa) level in cases with endometriosis and control (pg/ml)
| Cytokines (pg/ml) | Cases (n=37) | Controls (n=25) | Statistical analysis | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ‘t’ value | ‘p’ value | |
| IL-6 | 8.15 | 5.90 | 7.89 | 5.93 | -0.170 | 0.866 |
| IL-8 | 0.95 | 0.52 | 0.47 | 0.95 | -4.202 | <0.001 |
| TNF-a | 48.33 | 17.66 | 39.87 | 19.12 | -1.789 | 0.079 |
| Severity | IL-8 (<0.78 pg/ml) | IL-8 (>0.78 pg/ml) | Statistical analysis | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | x2 | ‘p’ value | |
| Mild | 8 | 80.00 | 19 | 76.00 | 0.065 (df=1 | 0.799 |
| Moderate | 2 | 20.00 | 6 | 24.00 | ||
Table 6: Correlation of severity of endometriosis in cases (AFS classification) Vs TNF-a cut-off value
| Severity | TNF-alpha (<45.5 pg/ml) | TNF-alpha (>45.5 pg/ml) | Statistical analysis | |||
|---|---|---|---|---|---|---|
| Number | % | Number | % | x2 | ‘p’ value | |
| Mild | 9 | 75.00 | 18 | 78.26 | 0.048 (df=1) | 0.827 |
| Moderate | 3 | 25.00 | 5 | 21.74 | ||
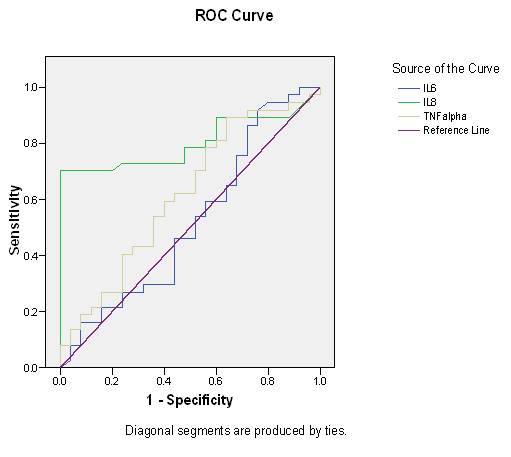
Fig. 1: ROC of serum Interleukin 6, Interleukin 8, TNF-a for prediction of endometriosis
Table 7: Area under Curve: Continuation of the above Fig and it’s the description of AUC for all three test marker
| Test Result Variable(s) | Area | Std. Error (a) | Asymptotic Sig. (b) | Asymptotic 95% Confidence Interval | |
|---|---|---|---|---|---|
| Lower Bound | Upper Bound | ||||
| IL6 | .520 | .078 | .791 | .368 | .672 |
| IL8 | .803 | .057 | .000 | .690 | .915 |
| TNF-alpha | .617 | .074 | .119 | .473 | .762 |
The test result variable(s): IL6, IL8 has at least one tie between the positive actual state group and the negative actual state group. Statistics may be biased.
a: Under the nonparametric assumption; b: Null Hypothesis:
True area = 0.5
For IL-6 Area under curve = 0.520 which is less than 0.7 hence no cut-off was explainable.
For IL-8 area under curve = 0.803 (good discriminant ability) cut off >0.78 pg/ml. It was 70.3% sensitive and 80% specific.
For TNF-a, area under curve= 0.617 (average discriminant ability) cut off >45.5 pg/ml which was 62.2% sensitive and 56% specific.
Table 8: Performance of Serum Cytokines (IL8, TNFa) for nonsurgical prediction of endometriosis
| Serum Markers | Cut off Point | Sensitivity (%) | Specificity (%) | Positive likelihood | Negative Likelihood |
|---|---|---|---|---|---|
| (pg/ml) | ratio | Ratio | |||
| IL8 | 0.78 | 70.3 | 80.00 | 3.51 | 0.37 |
| TNFa | 45.50 | 62.2 | 56.00 | 1.41 | 0.68 |
Serum IL-8 at cut off point of 0.78 pg/ml provided a sensitivity of 70.3%, specificity of 80%, and a positive likelihood ratio of 3.51% and negative likelihood ratio of 0.37%. Serum TNF-a at cut off point of 4.50 pg/ml provided a sensitivity of 62.2%, specificity of 56%, a positive likelihood ratio of 1.41% and negative likelihood ratio of 0.68%.
DISCUSSION
Endometriosis is suspected when a patient presents with chronic pelvic pain, dysmenorrhea, dyspareunia, or infertility. The gold standard for the diagnosis of endometriosis is laparoscopic inspection, ideally with histological confirmation [13]. The diagnosis of this disease depends on diagnostic laparoscopy, which is minimally invasive, but expensive, and associated with potential complications. There is a 2.4% risk of bladder or intestinal injury, two-thirds of which will require laparotomy. In addition, injury to a major blood vessel during laparoscopy can be catastrophic and has a reported mortality rate of 15% [14].
In our study group majority of subjects belong to age group 25-30 years. It indicates that subjects included in the study as controls were having significantly higher (p<0.001) age (30.88+2.28 years) as compared to cases (24.65+6.46 years) (Table 1).
secondary dysmenorrhea is endometriosis, which can be visually confirmed by laparoscopy in approximately 70% of adolescents with dysmenorrhea. Our study was supported by Ballard et al. [16] endometriosis is the most frequent cause of deep dyspareunia, and patients with the disease have a 9-fold increase in risk of experiencing this symptom when compared with the general female population .
In present study 56.7% subjects (Table 2) had infertility (both primary and secondary), it was advocated by Tomassetti et al. [17] that endometriosis can lead to anatomical distortions and adhesions (the fibrous bands that form between tissues and organs following recovery from an injury).
On pelvic examination, the target indications of women with endometriosis differ as indicated by the area and size of injury. One might discover to mass in adnexa, knobs in pocket of Douglas, cervical delicacy. It appears that in our concentrate all patients with unmistakable rectovaginal knob had endometriosis. A study was done by Matorras et al. [18], who reasoned that endometriomas might be identified as delicate or nontender adnexal masses, frequently settled to the uterus or to the pelvic sidewall. Delicate masses, knobs, and fibrosis might be acknowledged on palpation of the upper vagina, parkway, uterosacral ligaments, or rectovaginal septum. For a situation controlled study, the main indications of endometriosis in fruitless patients were uterosacral nodularity and uterosacral tenderness.
In our study based on USG finding (Table 3) we found that 21.62% cases had B/L ovarian endometrioma, 18.92% cases had endometriosis of pouch of Douglus, but the non-invasive approaches such as ultrasound, magnetic resonance imaging or blood tests have not yielded sufficient power for the diagnosis of endometriosis [19]. IL-6 is a T cell-derived cytokine that is secreted by macrophages, lymphocytes, ?broblasts, and endothelial cells. It has B cell stimulatory activity and enhances the differentiation of T lymphocytes. IL-6 is exceedingly associated with angiogenesis and migration. Inflammatory stimuli, IL-1ß and TNF-a, induces activin, which is a protein expressed in endometrioma stromal cells. It turns on the expression of IL-6 and PAR-2 mRNA and enhances the proliferation of endometrioma stromal cells. [20]. IL-8 may go about an autocrine growth factor in the endometrium which promotes the vicious circle of endometrial cell attachment, cell growth, and further self-stimulation in the pathogenesis of endometriosis. [21] Chemokine IL-8 expression in endometrial endothelial cells is stimulated by sex steroids in women with endometriosis. [22] IL-8 enhances endometrial stromal cell metalloproteinase activity and invasive capability that degrade extracellular matrix to assist the endometrial cells invade the peritoneum and to built up an endometriotic lesion. [23]
In our study level of serum IL-6 was higher in cases than controls but difference was statistically non-significant (Table 4). So the serum of subjects with endometriosis con?rm that this pleiotropic cytokine plays an important role in the pathogenesis of endometriosis both locally and systemically. Our finding was similar to study done by Somigliana et al. [19].
In our study, we found that serum IL-8 levels were significantly higher (p<0.001) in cases (0.95+0.52) as compared with controls (0.47+0.28) (Table 4), and for IL-8 area under curve = 0.803 (good discriminant ability) cut off >0.78 pg/ml (Fig-1). It was 70.3% sensitive and 80% specific for endometrial diagnosis, Carmona et al. [24] who found a high positive correlation between serum IL-8 levels in the endometriosis group. Serum IL-8 alone achieved the highest predictive value of the presence of endometriosis (OR: 1.44; sensitivity: 78.2%; specificity: 76.2%), thus it may become useful tools for discriminating endometriosis.
In this study, we found that serum TNF-? and were of higher order in Cases than in controls (Table 4) but this difference was statistically non-significant (p>0.05), which is contradictory to study of Bedaiwy et al. [25] who found a significantly high level of serum TNF-a levels in endometriosis group than the control group (P=0.003). In our study, 78.3% cases had mild (stage II) endometriosis and 21.6% cases had moderate (stage III) endometriosis. A study done by Essam et al. [26] recruited 68 patients with endometriosis of whom 32 were early stage disease (stage I and II) and 36 advanced stage endometriosis (stage III and IV). Wachyu [27] included 40 patients with endometriosis. Out of which 26 were classified as stage I and II and the remaining were classified as stage III- IV.
According to study by D’Hooghe et al. [28], a diagnostic test with sensitivity as high as 100% would be ideal even if the specificity is only 50%. In our study IL-8 had 70.3% sensitivity and 80% specificity and TNF-a had 62.2% sensitivity and 56% specificity (Table 6, Fig. 1). In present study we found that 76% cases of mild (stage II) endometriosis had IL-8 >0.78pg/ml (cut-off), and 24% cases of moderate (stage III) endometriosis had IL-8>0.78pg/ml. Regarding TNF-a, 78.26% cases of mild (stage II) endometriosis had TNF-a>45.5 pg/ml (cut-off) and 21.74% cases of moderate (stage III) had TNF-a >21.74% which did not show any significant association between stage of endometriosis and cut-off value of cytokines (Table 6). Wachyu [27] was also found that there was no significant difference in mean serum biomarkers level of IL-6, TNF-a, and between endometriosis stage I-II and stage III-IV (1.58 ± 0.78 vs 1.55 ± 0.98 pg/mL, 1.5 ± 0.47 vs 1.49 ± 0.29 pg/ml respectively). Othman et al. [29] concluded that level of serum IL-6 was higher in cases of endometriosis but there was no statistical significant difference between subjects with cases of endometriosis and controls. On contrary our study shows TNF-a and IL-8 was undetectable in cases and controls.
CONCLUSIONS
In the present study, we have observed that serum markers (IL-6, IL-8, TNF-a) levels are raised in cases of endometriosis. But IL-8 had 70.3% sensitivity and 80% specificity, so that it has good discriminate ability. TNF-a has average discriminate ability and has 62.2% sensitivity and 56% specificity so that serum IL-8 and TNF-a can differentiate cases with or without endometriosis. By detecting these serum markers, we can diagnose endometriosis without undergoing laparoscopy or laparotomy. Thus the serum markers (IL-8, TNF-a) can be taken as non-invasive tool for diagnosis of endometriosis.
ACKNOWLEDGMENT
We are indebted to Dr. Abbas Ali Mahdi, Head, Department of Biochemistry, KGMU who act as promoter at this research.
REFERENCES
| International Journal of Life-Sciences Scientific Research (IJLSSR)
Open Access Policy
Authors/Contributors are responsible for originality, contents, correct
references, and ethical issues.
IJLSSR publishes all articles under Creative Commons
Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |
| How to cite this article: Deo S, Jaiswar SP, Shankhwar PL, Iqbal B, Jhirwar M: Peripheral Biomarkers as Predictive Indicators of Endometriosis: A Prospective Case Control study. Int. J. Life. Sci. Scienti. Res., 2017; 3(5):1370-1376. DOI:10.21276/ijlssr.2017.3.5.18 Source of Financial Support:Nil, Conflict of interest: Nil |