ABSTRACT- Transient Receptor Potential Canonical (TRPC6) assumes vital part in pathophysiology of DN and is up-regulated by angiotensin II, high glucose level, Transforming growth factor beta (TGFß), and intercede podocyte damage in Diabetes Mellitus focusing on TRPC6 may reduce podocyte damage and proteinuria. From different investigation and proof gave by analyst and author work on pathophysiological part of TRPC 6 and the medications which modify or restrain TRPC6 or its downstream molecular target propose that TRPC6 is novel molecular target. Recently distinguished ROS/TRPC6 pathway will cover the best approach to new, reassuring restorative systems to target kidney ailments, especially Diabetic Nephropathy.
Key-words- Diabetic nephropathy, TRPC6, Podocyte Injury, Proteinuria
INTRODUCTION
Transient receptor potential (TRP) channels are a large family of proteins with six main subfamilies named as TRPC (canonical), TRPV (vanilloid), TRPM (melastatin), TRPP (polycystin), TRPML (mucolipin), and TRPA (ankyrin) sets [1].
Classification on the basis of Amino acids:
Mammalian TRP channel proteins form six transmembrane cation-permeable channels that may be clustered into six subfamilies on the basis of amino acid arrangement (TRPC, TRPV, TRPM, TRPA, TRPP, and TRPML) [2]. Many TRPs are expressed in kidney along diverse parts of the nephron and growing confirmation propose that these channels are tangled in hereditary, as well as acquired kidney disorders [2]. The total number of different TRPs with diverse functions supports the announcement that these channels are tangled in a wide range of processes ranging from distinguishing of thermal and chemical signals to reloading intracellular stores after responding to an extracellular stimulus. Mutations in TRPs are associated to pathophysiology and specific diseases [1]
Pathophysiological role of Transient Receptor Potential (TRP) Channels:
TRP melastatin (TRPM2) non-selective cation channels are expressed in the cytoplasm & intracellular organelles,
where their inhibition ameliorates ischaemic renal pathology [3]. TRPV4 may function as an osmoreceptor in kidney and participate in the regulation of sodium and water balance [2]. TRP vanilloid 4 receptor channels (TRPV4) are highly expressed in the kidney, where they induce Ca2+ influx into endothelial and tubular cells. [3] TRPV5 contributes to several acquired mineral dysregulation, such as diabetes mellitus (DM), acid-base disorders, diuretics, immunosuppressant agents, and vitamin D analogues-associated Ca2+ imbalance [2]. TRPC6, TRPM6, and TRPP2 have stayed associated in hereditary focal segmental glomerulosclerosis (FSGS), hypomagnesemia with secondary hypocalcemia (HSH), and polycystic kidney disease (PKD) [3]. Transient receptor potential cation channel, subfamily C, member 6 (TRPC6) in podocytes is tangled in chronic proteinuric kidney disease, mainly in focal segmental glomerulosclerosis (FSGS) [3]. TRPM6, located mainly in distal convoluted tubules, appears to be a vulnerable molecule that causes hypermagnesiuric hypomagnesemia as a tubulointerstitial nephropathy-independent altered tubular function in diabetic nephropathy [4].
Role in Kidney: Associates of the transient receptor potential (TRP) cation channel receptor family have unique sites of regulatory function in the kidney which allows them to promote local vasodilatation and controlled Ca2+ influx into podocytes and tubular cells [3].
Renoprotection
Animal Study: Triggered TRP vanilloid 1 receptor channels (TRPV1) have been found to elicit renoprotection in rodent models of acute kidney injury subsequent ischaemia/reperfusion. [3]
TRPC6: Canonical transient receptor potential 6 (TRPC6) proteins accumulate into hetero-multimeric arrangements forming non-selective cation channels. TRPC6- interacting proteins have been recognized like some enzymes, channels, pumps, cytoskeleton-associated proteins, immunophilins, or cholesterol-binding proteins, demonstrating that TRPC6 are involved into macromolecular complexes [5].
Pathogenesis role: TRPC proteins in the progress of various diabetic complications, such as diabetic nephropathy and diabetic vasculopathy [6]. TRPC-mediated effects on podocytopenia in DN initiation [7]. The physiological role of podocytes is depreciatively dependent on proper intracellular calcium handling; undue calcium influx in these cells may result in the effacement of foot processes, apoptosis, and subsequent glomeruli damage. One of the key proteins accountable for calcium flux in the podocytes is transient receptor potential cation channel, subfamily C, member 6 (TRPC6) [7]. Slit diaphragm and podocyte damage is key in the pathogenesis of proteinuria in diabetic nephropathy (DN). Gain-of-function mutations in TRPC6, a slit diaphragm-associated ion channel, cause glomerulosclerosis; TRPC6 expression is amplified in acquired glomerular disease [8]. Podocyte depletion may result from improper calcium handling due to abnormal opening of the calcium permeant TRPC (Transient Receptor Potential Canonical) channels [7].
ROS production and TRPC6: Both overproduction of ROS and dysfunction of TRPC6 channel are tangled in renal injury in animal models and human subjects [6].
Mutation in TRPC6: Mutation in TRPC6 has been connected with the commencement of the familial forms of focal segmental glomerulosclerosis (FSGS) [7].
Diabetes and TRPC6: Angiotensin II (Ang II) levels are found to be higher in diabetes [9] Ang II causes stimulation of TRPC6 in podocytes [9].
TRPC 6 activation and Podocyte injury: The second messenger diacylglycerol, store-depletion, the plant extract hyperforin or H2O2 have all been revealed to trigger the opening of TRPC6 channels. A well-characterized importance of TRPC6 activation is the elevation of the cytosolic concentration of [(Ca2+)]i [5]. Ang II causes stimulation of TRPC6 in podocytes [9]. Glucose can trigger a local renin-angiotensin system in the podocyte, leading to amplified TRPC6 expression, which enhances TRPC6-mediated Ca(2+) influx. Regulation of TRPC6 expression could be a vital factor in podocyte injury due to chronic hyperglycemia [8].
High Glucose: Induced apoptosis and reduced viability of differentiated podocytes. It caused time-dependent up-regulation of TRPC6 and stimulation of the canonical Wnt signalling pathway, in mouse podocytes [10]. The transient receptor potential channel 6 (TRPC6) is a vital Ca2+ permeable ion channel in podocytes, which is tangled in high glucose (HG)-induced podocyte apoptosis. [11].
Regulation by VGEF: VEGF controls TRPC6 in podocytes [12]. Increased plasma concentrations of vascular endothelial growth factor (VEGF) and amplified expression of transient receptor potential canonical type 6 (TRPC6) channels in podocytes have been linked with proteinuric kidney diseases [12].
Effect of TRPC Activation: TRPC6 channel stimulation increased [(Ca2+)]i concentration, inhibited proliferation, and triggered apoptotic cell death in primary neonatal pig GMCs (Glomerular mesangial cells) [13].
TRPC6 signal plays a key role in mediating TGF-ß1 induced podocyte injury via nephrin, desmin and caspase-9. [14]
Pathway: GPCRs coupled to Gq signaling activate TRPC6, proposing that Gq-dependent TRPC6 stimulation underlies glomerular diseases [15]. Wnt/ß-catenin signalling pathway may possibly be dynamic in pathogenesis of TRPC6-mediated diabetic podocyte injury [10]. TRPC6 channel-dependent [(Ca2+)]i rise and the later induction of the calcineurin/NFAT, FasL/Fas, and caspase signalling cascades promote neonatal pig GMC apoptosis [13]. TGF-ß1 induced by glomerulosclerosis impairs the protein expression of nephrin and increases the protein expression of desmin and caspase-9 via TRPC6 signal pathway [14]. TRPC6 is a redox-sensitive channel, and variation of TRPC6 Ca2+ signalling by altering TRPC6 protein expression or TRPC6 channel activity in kidney cells is a downstream mechanism by which ROS bring renal damage [20].
Drugs that inhibit TRPC6: FK506 could ameliorate podocyte injury in T2DM, which may be linked to suppressed expressions of TRPC6 and NFAT [16]. Angiotensin receptor blockade and inhibition of local AngII production through angiotensin-converting enzyme inhibition prevented glucose-mediated intensified TRPC6 expression [8]. Calcitriol can ameliorate podocyte injury, which is contributed by the inhibition of enhanced TRPC6 expression in the primary stages of DN rats [17]. Astragaloside IV (AS-IV) is a saponin sequestered from Astragalus membranaceous, which possesses various pharmacological activities. AS-IV protected HG-induced podocyte apoptosis, down regulated TRPC6 expression and blocked intracellular Ca2+ in HG-stimulated podocytes. AS-IV also suppressed NFAT2 and Bax expression [11]. AS-IV may check HG-induced podocyte apoptosis via down regulation of TRPC6, which is maybe mediated via the calcineurin/NFAT signaling pathway [11]. Blockade of Wnt/ß-catenin signalling by paricalcitol ameliorates proteinuria and kidney injury [18]
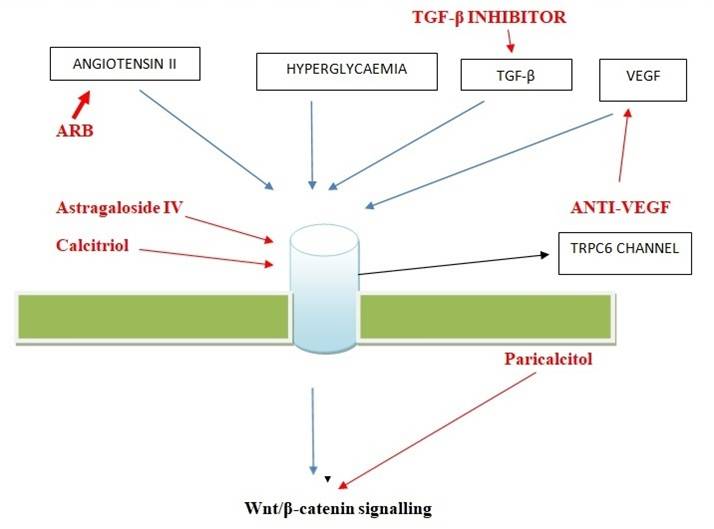
Blue Line-: Up Regulators of TRPC6
Red Line-: Inhibitor drugs and targeting downstream pathway
Targeting: Targeting TRPC6 or downstream signalling molecules or pathway may help in DN i.e. Targeting Gq/TRPC6 signalling may have therapeutic benefits for the treatment of glomerular diseases [15]. Blocking TRPC6 signal pathway has a protective effect on podocyte injury [14]. VGEF [14] is regulator of TRPC6 in podocytes [12]. VGEF Inhibitors-OPB-9195 [21], an AGE-inhibitor, prevented the development of diabetic nephropathy by hindering type IV collagen production and suppressing overproduction of two growth factors, TGF-ß and VEGF, in diabetic rats, this compound needs additional investigation [19]. Newly identified ROS/TRPC6 pathway will cover the way to new, encouraging therapeutic strategies to target kidney diseases such as diabetic nephropathy [20]. Wnt/ß-catenin signalling pathway [10] may potentially be active in pathogenesis of TRPC6-mediated diabetic podocyte injury [10]. Blockade of Wnt/ß-catenin signaling by paricalcitol [14] ameliorates proteinuria and kidney injury [18].
From various study and evidence provided by researcher and author on pathophysiological role of TRPC 6 and the drugs which modify or inhibit TRPC6 or its downstream signalling molecules which suggest that TRPC6 is novel molecular target.
CONCLUSIONS
TRPC6 assumes vital part in pathophysiology of Diabetic nephropathy (DN) and is up-regulated by way of Angiotensin II, Hyperglycaemia, TGFß and intercede podocyte damage in Diabetes Mellitus that attract attention towards TRPC6, targeting might reduce podocyte damage and proteinuria. From unique research and evidence gave by expert and author work on pathophysiological role of TRPC 6 and the medicinal drugs which regulate or restrain TRPC6 or its downstream molecular target propose that TRPC6 is unique molecular target. Future days will distinguish ROS/TRPC6 pathway, which will cover the quality method to new, reassuring restoration of kidney structures specially Diabetic Nephropathy.
ACKNOWLEDGMENT
To all Researchers, Scientist, Scholar who has honestly and sincerely dedicated their work for Nobel causes and there are no words to express my gratitude. I dedicated the article to all Authors [1-22] in bibliography.
REFERENCES
| International Journal of Life-Sciences Scientific Research (IJLSSR)
Open Access Policy Authors/Contributors are responsible for originality, contents, correct references, and ethical issues. IJLSSR publishes all articles under Creative Commons Attribution- Non-Commercial 4.0 International License (CC BY-NC). https://creativecommons.org/licenses/by-nc/4.0/legalcode |
| How to cite this article: Soni NO: TRPC 6 as a Molecular Target in Diabetic Nephropathy. Int. J. Life. Sci. Scienti. Res., 2017; 3(5):1311-1314. DOI:10.21276/ijlssr.2017.3.5.8 Source of Financial Support:Nil, Conflict of interest: Nil |