Bactericidal activity of Flavonoids
isolated from Muntingia calabura
Srinivas Gorripati1*, Konka Rajashekar2, Deepa
Dasu3, Anvesh Jupaka4, Murali Krishna Thupurani5
1Research Scholar, Department of Biotechnology, Krishna University, Machilipatanam, Andhra Pradesh, India
2,4,5 Department of Biochemistry, Chaitanya Degree College (Autonomous), Kakatiya
University, Telangana, India
3SRF, Clinical division, (National Institute of Nutrition), NIN Hyderabad,
India
*Address for correspondence:
Mr. Srinivas Gorripati,
Research Scholar, Department of Biotechnology, Krishna
University Machilipatanam, India
ABSTRACT- The investigation was carried out for the isolation and characterization
of the compounds from heart wood of root and root bark of Muntingia calabura. We have isolated six compounds, three from
each extract and were identified as flavonoids. The bactericidal activity of
these compounds found significant against tested bacterial strains. Among the
tested compounds, 8-methoxy,3ʹ,5ʹ7ʹ-trihydroxyflavone and 3,5,7-trihydroxyflavone
(Galangin) showed paramount activity against MRSA. The
results are compared with known standards gentamycin sulphate and cefixime.
Key words- Muntingia calabura, Bactericidal, broth dilution method, MRSA, Flavonoids
INTRODUCTION- Flavonoids are polyphenolic
secondary metabolites, ubiquitously found in nature. Over 4,000
flavonoids have been identified from different sources. The
potential therapeutic applications of these metabolites have been considerable
interest in recent years [1-4]. Antibacterial resistance a “ticking
time bomb” of public heath, serious threatening issue, when eve a simple
infection turns to fatal and if tomorrow it extends its current course could
become even worst. Majority of the plant
metabolites in drug discovery has come from the diverse structures of the
medicinal plants. These are often perceived as immense drug-likeness and more
biological friendliness and making them good candidates in drug development [5-9].
Muntingia calabura is native to Southern Mexico and
Central America, distributed all over the tropical regions of the world and
especially, In India. Muntingia calabura
crude extracts for the treatment of various human disorders requires a proper
scientific evaluation and documentary reports of active principle responsible [10,11]. Several researchers round the globe have been
isolated and identified the compounds of this plant as flavonoids [12-14].
Till the date, only few of these compounds have been evaluated for its
therapeutic properties and still there are several compounds stand remained for
scientific evidence based utilization [15,16].
Thus, the compounds isolated in current study have been further determined for
their attributed biological activity. With regard microbial resistance and
plant-derived drugs, the current investigation has been documented about
bactericidal activity of flavonoids isolated from heart wood root and root bark
of Muntingia calabura.
MATERIALS AND METHODS
Plant Material- Heart wood of root and root bark of Muntingia calabura was collected from College premises of Chaitanya Degree and Postgraduate College (Autonomous), Hanamkonnda, Warangal District, Telangana, India. The authenticity of the plant was carried out by Prof. V.S. Raju, Taxonomist, Plant systems laboratory, Department of Botany, Kakatiya University, Warangal, India (Voucher number: Dep/B/KU/WGL/MC-014/2013). The plant material was chopped into smaller fragments, dried under shade and grinded in homogenizer to coarse powder.
Chemicals used- All the chemicals required for media preparation and bactericidal assay were purchased from Hi-media chemical laboratories, Mumbai, India and are of analytical grade.
Extraction and separation of compounds - The plant material was finely powdered (500 g) and extracted with chloroform in a soxhlet apparatus. The extract was concentrated under reduced pressure. The resultant gummy product was further used for separation of compounds by column chromatography.
Bacterial Strains and their Growth- “Gram-positive” strains Methcillin-resistant Staphylococcus aureus NCTC 13616, Bacillus subtilis ATCC 6633, Bacillus cereus, ATCC 14579 and “Gram-negative” strains Klebsiella pneumoniae ATCC 43816, Escherichia coli ATCC 8739, Proteus vulgaris ATCC 13315 were procured from American type culture collection, USA. Methicillin-resistant Staphylococcus aureus was purchased from culture collections, UK. All bacterial strain stored at -80oC were streaked on Luria-Bertani (LB) agar plates (Hi-media Laboratories, Mumbai, India) and incubated at 37oC for 20 to 24 h. A few isolated colonies were harvested from each plate and suspended in 5 ml of LB broth contained in a 15 ml of sterile plastic tube. The tube was capped tightly and incubated with gentle shaking (140 rpm) at 37oC for 20 h.
Preparation of bacteria for bactericidal assay- Broth culture (1 ml) of
test organisms was added separately to a 1.9 ml eppendorf
tube, bacterial sedimentation was achieved by centrifugation at 12,000 rpm for
30 sec. The pellet was re-suspended using 1 ml of sterile PBS by gentle
aspiration in and out of a transfer pipette. The optical density (OD) of the
pellet was determined at 620 nm in spectrophotometer. The OD at 620 of the
sample was adjusted approximately to 0.8 to 0.9 by the addition of PBS. Ten microliters of the diluted sample was subjected for serial
dilution with PBS so that these dilutions would produce approximately 1,500 to
2,000 bacteria per 50ml sample. The ODs of the samples results in 60 to 200
CFU/mL.
Preparation of compound stocks
and their dilutions- 10,000 mg of each isolated compound was dissolved in one liter of PBS. Further, 1 mL of this solution was diluted in 9 mL
of PBS to generate 1000 mg/L stock. This stock was used for serial dilutions to
produce the concentrations ranging from 0.1 - 200 mg/mL.
Cefixime
and Gentamycin sulphate are
used as positive control (10µg/L). Solubility was achieved by adding few drops
of saturated NaHCO3. The dilution of the compounds was achieved by
dissolving 1 mg of compound in 1L of WFI (Water for Injection). One mL of this dilution was dissolved in 9 mL
of WFI to produce 10 µg/L concentrations and used in the study.
Bactericidal assay- The assay was conducted to assess the bactericidal activity
of the isolated compounds through microtiter plate
described previously. The assay reaction mixture consisted of PBS (50mM
sodium phosphate, 150 mM NaCl
[pH 7.0]), test compounds at various concentrations
and the bacterial strains were prepared in sterile 96-well microtiter
plates (Nunc, Inc). The wells are filled with 100µl
diluted test compounds in PBS and 50 µl of the diluted bacterial strains and
incubated with gentle shaking (140 rpm) at 37oC for various
incubation periods [0 (baseline), 2, 4, 8, 12,
and 24 h) (time-kill studies)] 24 h. Subsequently, positive and negative
controls, was prepared and screened. Following incubation, a 20µl aliquot from
each well was spotted at the top of a square plate containing Nutrient agar
medium. The plate was labeled and tapped gently to facilitate the movement of
the liquid. There were approximately 200 cells in the spotted (20 µl) sample. Plates were placed uncovered
in biohood until the sample liquid dried (ca. 10 min)
and incubated overnight at 37oC. CFU of test organisms were visible after 18 to 24 h and was counted.
The experiments were performed in duplicate, and CFU for each streak were
enumerated with a colony counter.
The control value to determine the percentage of bacteria
killed per well. The percentage of the bacteria killed was plotted graphically,
and the percentage of the test compound resulting decrease in the number of CFU
at each dilution of test compounds was compared with the average of positive
number of CFU (BA50) was determined.
RESULTS
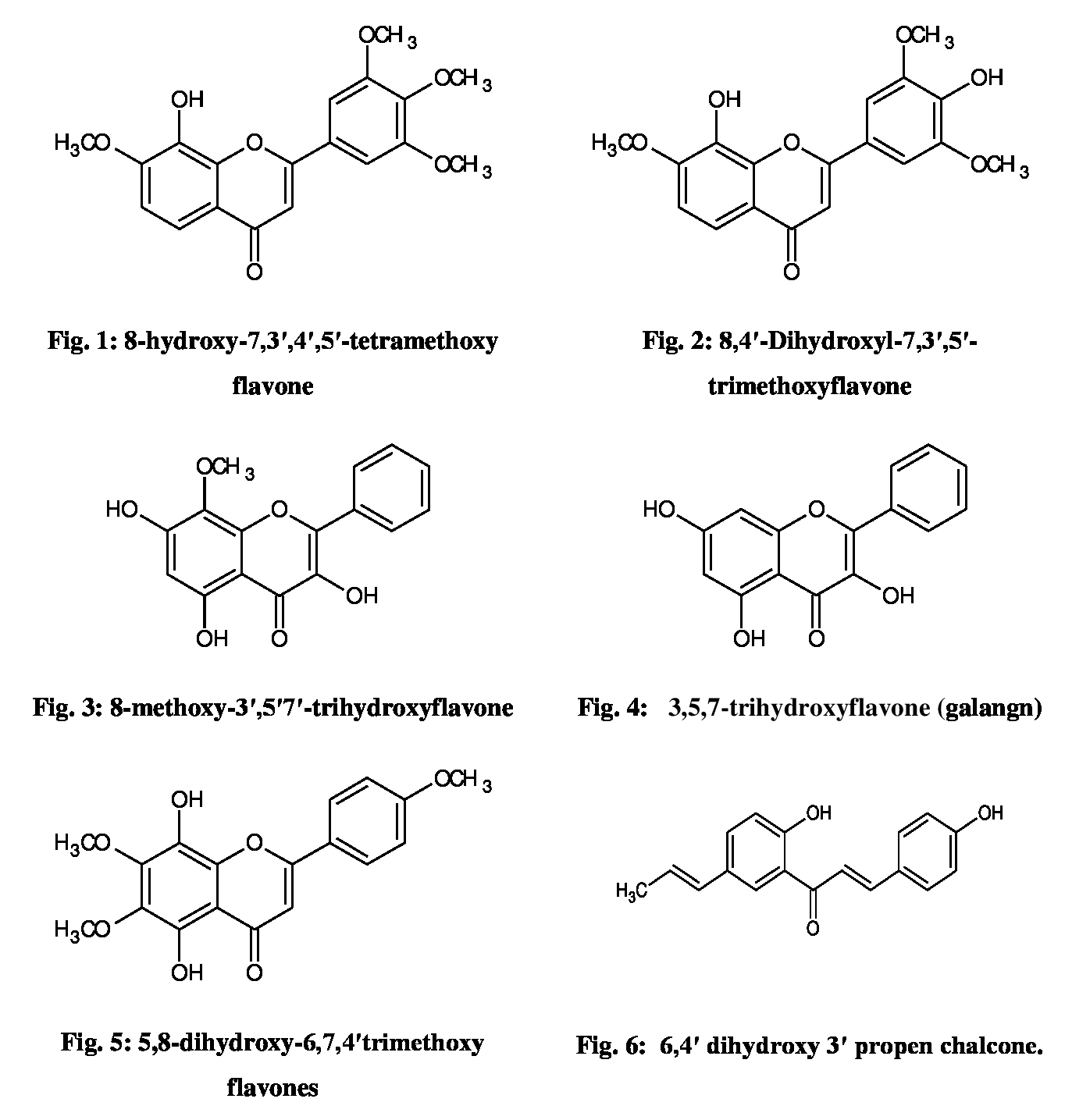
Characterization
of isolated compounds- We have been
isolated and identified five compounds as flavones and one compound as Chalcone
and characterized by spectral data (1HNMR, 13C and Mass)
as 8-hydroxy,7,3ʹ,4ʹ,5ʹ-tetramethoxy flavone (Fig. 1); 8,4ʹ-
Dihydroxy,7,3ʹ,5ʹ-trimethoxyflavone (Fig.
2); 8- methoxy,3ʹ,5ʹ7ʹ-trihydroxyflavone (Fig. 3); 3,5,7-trihydroxyflavone
(Galangin) (Fig. 4); 5,8-dihydroxy,6,7,4ʹ-trimethoxy flavones (Fig. 5); 6,4ʹ-dihydroxy,
3ʹ-propen chalcone (Fig. 6) (Table 1). Based on mass spectra the compounds possess the
molecular weight and molecular formula m/z
358.34202, C19O7H18 (Fig. 1); m/z 344.31544, C18O7H16
(Fig. 2); m/z 300.26288, C21H20O4
(Fig. 3); m/z 270.2369, C21H25O4
(Fig. 4); m/z 344.31544, C18H16O7
(Fig. 5); m/z 280.109396, C18H16O3
(Fig-6) respectively. The structures of the isolated compounds were
depicted in Fig. 1 - 6.
Table 1: 1HNMR
data of the isolated compounds from heart wood of root and root bark of Muntingia
calabura
|
Compound-5 |
Compound-6 |
|||||||
|
1H(δ in ppm) |
13 C (δ n ppm) |
1H(δ in
ppm) |
13 C (δ n ppm) |
|||||
|
1 |
- |
120.03 |
||||||
|
2 |
- |
163.01 |
2 |
7.60 (S) |
130.2 |
|||
|
3 |
6.53 (S) |
102 |
3 |
- |
132.78 |
|||
|
4 |
- |
184.01 |
4 |
6.68 (d) |
130.42 |
|||
|
5 |
12.69 (-OH, S) |
151.6 |
5 |
6.72 (d) |
123.45 |
|||
|
6 |
4.02 (OCH3, S) |
130.01, 56.6(OCH3) |
6 |
5.22 (OH, S) |
160.69 |
|||
|
7 |
3.96(OCH3, S) |
158.06, 5 6.2 (OCH3) |
7 |
- |
170.2 |
|||
|
8 |
8.61(OH, S) |
128.3 |
Α |
7.43 (d) |
126.45 |
|||
|
9 |
- |
139.9 |
Β |
7.01 (d) |
147.32 |
|||
|
10 |
- |
108.6 |
1’ |
- |
128.63 |
|||
|
1’ |
- |
125.8 |
2’,6’ |
7.70 (d) |
131.62 |
|||
|
2’,6’ |
7.91(d, J=8.7Hz) |
129.3 |
3’,5’ |
7.72 (d) |
117.02 |
|||
|
3’,5’ |
7.01 (d, J= 8.7 Hz) |
114.01 |
4’ |
5.52 (OH, S) |
162.03 |
|||
|
4’ |
3.91(OCH3, S) |
161.6, 55.6 (OCH3) |
1’’ |
4.58 (d) |
132.04 |
|||
|
2’’ |
4.48 (M) |
124.72 |
||||||
|
3’’ |
1.29 (d) |
19.23 |
||||||
Bactericidal assay
8-hydroxy,7,3ʹ,
4ʹ,5ʹ-tetramethoxy flavone
(Compound- 1)- The susceptibility nature
of test strains against compound 8- hydroxy,7,3ʹ,4ʹ,5ʹ-tetramethoxy flavone was found in
concentration dependent manner and exhibited significant bactericidal activity
against Bacillus cereus, Bacillus subtilis, E. coli and Proteus vulgaris
with 79,73, 66 and 62 bactericidal percentages respectively. Klebsiella pneumoniae found slightly
resistant and noticed 50% death percentage (Table 2).
4ʹ-dihydroxy,7,3ʹ,5ʹ-trimethoxy flavone (Compound-2)- 4ʹ-Dihydroxy,7,3ʹ,5ʹ-trimethoxy flavone was found very active against MRSA and
Bacillus subtilis, E. coli, Klebsiella pneumoniae and Proteus vulgaris. The bactericidal rates
were found at 1.0 mg/mL is 71, 70, 71, 52 and 53
respectively. Bacillus cereus
exhibited average susceptibility with 44 bactericidal death rates at 0.7 mg/mL (Fig. 3 and Table 2).
8-methoxy,3ʹ,5ʹ7ʹ-trihydroxyflavone
(Compound-3)- 8-methoxy,3ʹ,5ʹ7ʹ-
trihydroxy flavone noticed
highest bactericidal activity comparing to other compounds. The highest
bactericidal percentages 94, 89, 77, 96 and 80 are noted at 1.0 mg/mL against MRSA, Bacillus
subtilis, Bacillus cereus, E. coli and Proteus
vulgaris respectively.
The BA50 of this compound against MRSA was found
<1mg/mL (Table 2).
3,5,7-trihydroxyflavone or Galangin (Compound-4)- Basing
on the results the bactericidal activity of the compound was found against both
Gram positive and Gram negative strains. MRSA was showed high susceptibility
nature at all concentrations tested. The high bactericidal percentage 97 was
observed at 1.0 mg/mL. Galangin at 1.0 mg/mL showed moderate activity against Bacillus subtilis and Bacillus
cereus with 50 and 48 death percentages respectively. On the other hand,
among Gram negative strains, Proteus
vulgaris exhibited highest susceptibility 64% at 1.0 mg/mL
(Table 2).
5,8-dihydroxy,6,7,4ʹ-trimethoxyflavones (Compound-5)- Bactericidal efficacy of this compound was found effective
against MRSA, Bacillus cereus, Klebsiella
pneumoniae, Bacillus subtilis, E.
coli and Proteus vulgaris noticed
moderate activity (Table 2).
6,4ʹ-dihydroxy,3ʹ-propen
chalcone (Compound-6)- This compound is
most active against MRSA and E. coli.
The bactericidal percentage 85 and 76 were recorded against E. coli and MRSA respectively. Other
bacterial strains are exhibited moderate susceptibility (Table 2).
Effect
of incubation period on bactericidal activity- To determine the sensitivity of the bacterial strains, we
have performed time-kill studies, where the bacterial strains are incubated at
different incubation time periods with test compounds. During the study, we
have noticed that for most of the tested bacterial species, the susceptibility was
initiated after 4 hrs and some bacterial strains viz., MRSA, E. coli,
and Bacillus subtilis showed
susceptibility with 2 hrs of incubation. The bactericidal activity of some
compounds was found high for first 16 - 12 hrs and followed by plateau in
activity during the next 12-24 hrs. However, the MRSA count was started to
decrease after 2 hrs of incubation and the count was significantly reduced
during 8 - 12 hrs of incubation.
Table 2: Bactericidal
activity (BA50) of isolated compounds and standards against tested
bacterial strains
|
|
MRSA |
B. subtilis |
B. cereus |
E. coli |
K. pneumoniae |
P. vulgaris |
|
Compound-1 |
0.8 |
>0.7 |
>0.7 |
>0.6 |
>0.7 |
>0.6 |
|
Compound-2 |
>1.0 |
>1.0 |
>0.7 |
>0.6 |
>0.7 |
>0.6 |
|
Compound-3 |
<0.4 |
<0.4 |
0.6 |
>0.3 |
<1.0 |
>0.3 |
|
Compound-4 |
>0.5 |
<0.8 |
0.8 |
<0.7 |
<0.8 |
<0.7 |
|
Compound-5 |
0.5 |
>0.5 |
>0.6 |
>0.6 |
0.7 |
>0.6 |
|
Compound-6 |
>0.6 |
>0.7 |
>0.6 |
>0.4 |
>0.8 |
>0.7 |
|
Gentamycin |
<0.8 |
<0.6 |
0.5 |
<0.7 |
>0.9 |
0.8 |
|
Cefixime |
>0.6 |
<0.5 |
<0.4 |
<0.7 |
<0.7 |
0.6 |
DISCUSSION-
Isolated compounds, five flavonoids and
one structurally sub-set of flavonoid is chalcone (Fig. 1 - 6) are previously
reported. However, bactericidal activity of these compounds is documented here
for the first time. In accordance to the results obtained 8-methoxy,3ʹ,5ʹ7ʹ-trihydroxyflavone (Fig. 3), 3,5,7-trihydroxyflavone
(Fig. 4), 5,8-dihydroxy,6,7,4ʹ-trimethoxy flavones (Fig. 5) exhibited significant BA50
values against the bacterial strains tested especially, MRSA. Whereas, 8-
hydroxy,7,3ʹ,4ʹ,5ʹ-tetramethoxy flavone (Fig-1), 4ʹ-
Dihydroxy,7,3ʹ,5ʹ-trimethoxyflavone (Fig. 2), 6,4ʹ-dihydroxy, 3ʹ-propen chalcone (Fig.
6) recorded average inhibition effect on all strains used in the study. The
high susceptibility nature of MRSA might be attributed to dihydroxylation
of A ring at 5th and 7th positions on of 8- methoxy,3ʹ,5ʹ7ʹ-trihydroxyflavone
(Fig. 3), 3,5,7-trihydroxyflavone (Fig.
4) [17]. Inhibition of H+-ATPase-mediated
proton pumping could also establish the higher activity of these compounds
against MRSA [18]. In addition, the ability of 3,5,7-trihydroxyflavone (Fig.
4), to induce the damage of cytoplasmic membrane and
subsequent loss of potassium supported the destruction pathway of MRSA [19].
By Keen observations on destruction pathways of MRSA we also noticed that
isolated compounds are capable of interference with energy metabolism for the
inhibition of oxygen consumption by MRSA [20]. On the other hand,
the susceptibility nature of Gram negative strains Escherichia coli, Klebsiella
pneumoniae and Proteus vulgaris was also
found significant. This might be due to inhibition of DNA replication enzyme
DNA gyrase [21]. Generally, flavonoids exhibit biological activity by the
inhibition of eukaryotic enzymes [1]. In addition, the
ability of disruption and denaturation of cell wall
proteins by flavonoids add more value for bactericidal activity of the isolated
compounds [18]. It was reported that anti-bacterial activity assay
of flavones and chalcones isolated from leaf extracts
of Muntingia calabura possess significant activity [22]. As flavonoids are nonpolar and exhibit poor diffusion in agar gels [23],
antibacterial assays of flavonoids that relay on agar diffusion is not
suggestible, therefore, we studied using broth micro dilution method which is
more suitable for determining the bactericidal activity.
CONCLUSIONS- The nontoxic nature of flavonoids and
their attributed biological activities for prevention and treatment of wide
range of pathologies is drastically gained immense importance round the globe.
These are ubiquitous in plant kingdom and many of these are prescribed as
traditional medicine for thousands of years. In the current investigation, we
have investigated and given a detailed report on structural aspects and its
bactericidal activity of flavonoids isolated from Muntingia calabura. However, the study of flavonoids is perplexing
because of their molecular heterogeneity. In conclusion, we initially suggested
that the therapeutic strategies of flavonoids are an epitome for development of
effective future drugs against variety of bacterial infections. So many bacterial gets resistant to various
antibiotics. Owing to excessive usage of antibiotics the bacteria converted
into superbugs. Extendable research needed to discover novel flavonoids against
bacterial superbug and replaces the outmoded antibiotics. In this context there
is a need to develop research programmes on flavonoids against various
pathogens to develop human health and reduces the usage of antibiotics.
ACKNOWLEDGEMENTS- We thankful to Chaitanya Degree
College (Autonomous), Kakatiya University, Telangana, India for their supporting during the
entire work and extending their hands for accomplishing this work.
CONTRIBUTION OF AUTHORS- All authors were contributed equally for accomplishing this work.
REFERENCES
1. Havsteen B. Flavonoids, A class of natural products of high pharmacological potency. Biochem pharmocol, 1983; 32: 1141-8.
2. Middleton Jr E, Kandaswami C, Theoharides. The effect of plant flavonoids on mammalian cells. Implications for inflammation, Heart disease and cancer. The American society for pharmacology and experimental therapeutics, Pharmacol Rev., 2000; 52: 673-751.
3. Harbone JB, Baxter H. The handbook of natural flavonoids, Vol 1 and 2, Chichester, UK. John Wiley and Sons, 1999; pp. 1838.
4. Harborne JB, Williams CA, Advances in flavonoid research since 1992. Phytochemistry, 2000; 55: 481-504.
5. Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov., 2005; 4: 206-220.
6. Balunas MJ, Kinghorn AD. Drug discovery from medicinal plants. Life Sci., 2005; 78: 431-441.
7. Jones WP, Chin YW, Kinghorn AD. The role of pharmacognosy in modern medicine and pharmacy. Curr Drug Targets, 2006; 7: 247-264.
8. Drahl C, Cravatt BF, Sorensen EJ Cravatt BF. Protein-reactive natural products. Angew Chem Int Ed Engl, 2005; 44: 5788-5809.
9. Newman DJ, Cragg GM, Snader KM. Natural products as sources of new drugs over the period 1981-2002. J Nat Prod, 2003; 66: 1022-1037.
10. Sridhar
M, Thirupathi1 K, Chaitanya G, Kumar BR, Krishna
Mohan G. Antidiabetic effect of leaves of Muntingia calabura L., in normal and alloxan induced diabetic rats. Pharmacologyonline, 2011; 2: 626-632.
11. Zakaria ZA, Mohamed AM, Mohd. Jamil NS, Rofiee MS, Hussain MK, Sulaiman MR, The LK, and Salleh MZ. In vitro anti-proliferative, antioxidant activities of the extracts of Muntingia calabura leaves. Am J. Chin. Med., 2011; 39: 183-200.
12. Chen JJ, Lee HH, Duh CY. Cytotoxic chalcones and flavonoids from the leaves of Muntingia calabura. Planta med., 2005; 71: 970-973.
13. Sufian AS, Ramasamy K, Ahmat N, Zakaria ZA, Yusof MI. Isolation and identification of anti-bacterial and cytotoxic
compounds from the leaves of Muntingia
calabura L. J Ethnopharmacol, 2013; 146: 198-204.
14. Yusof M, Yusof IM, Salleh MZ, Kek TL, Ahmat N, Nik Azmin
NF, Zainul Zakaria AZ. Activity-guided isolation of bioactive
constituents with antinociceptive activity from Muntingia calabura L. Leaves using the formalin Test. Evidence-Based Complementary and
Alternative Medicine, 2013; pp. 1-9.
15. Dall Agnol R, Ferraz A, Bernardi AP, Albring D, Nor C, Sarmento L, Lamb L, Hass M, Von Poser G, Schapoval EE. Antimicrobial activity of some Hypericum species. Phytomedicine, 2003; 10: 511-6.
16. El-Abyad MS, Morsi NM, Zaki DA, Shaaban MT. Preliminary screening of some Egyptian weeds for antimicrobial activity. Microbios, 1990; 62: 47-57.
17. Tsuchiya H, Sato M, Miyazaki T, Fujiwara S, Tanigaki S, Ohyama M, Tanaka T, Iinuma M. Comparative study on the antibacterial activity of photochemical flavaonones against methicillin-resistant Staphylococcus aureus J. Ethnopharmacol, 1996; 50: 27-34.
18. Kuete V, Poumale Poumale HM, Guedem AN, Shiono Y, Randrianasolo R, Ngadjui BT, Antimycobacterial, anti-bacterial and antifungal activities of the methanol extract and compounds from Thecacoris annobonae (Euphorbiaceae). S. Afr. J. Bot., 2010; 76: 536–542.
19. Cushnie TP, Lamb AJ. Detection of galangin-induced cytoplasmic membrane damage in Staphylococcus aureus by measuring potassium loss, J Ethnopharmacol, 2005; 101: 243-8.
20. Haraguchi H, Tanimoto K, Tamura Y, Mizutani K, Kinoshita T. Mode of antibacterial action of retrochalcones from Glycyrrhiza inflata. Phytochemistry, 1998; 48: 125-9.
21. Mori A, Nishino C, Enoki N, Tawata S, Antibacterial activity and mode of action of plant flavonoids against Proteus vulgaris and Staphylococcus aureus. Phytochemistry, 1987; 26: 2231-4.
22. Zakaria ZA, Fatimah CA, Mat Jais AM, Zaiton H, Henie EFP, Sulaiman MR, Somchit MN, Thenamutha M, Kasthuri D. The In vitro anti-bacterial activity of Muntingia calabura extracts. International journal pharmacology, 2006; 2: 439-442.
23. Zheng WF, Tan RX, Yang L, Liu ZL. Two flavonones
from Artemisia giraldii and their
antimicrobial activity, Planta Med, 1996; 62: 160-2.